Abstract
The only dipeptide found to serve as a leucine source for a Salmonella strain lacking peptidases N, A, B, D, P, and Q was alpha-L-aspartyl-L-leucine. A peptidase (peptidase E) that specifically hydrolyzes Asp-X peptides was identified and partially purified from cell extracts. The enzyme (molecular weight, 35,000) is inactive toward dipeptides with N-terminal asparagine or glutamic acid. Mutants (pepE) lacking this enzyme were isolated by screening extracts for loss of the activity. Genetic mapping placed the pepE locus at 91.5 map units and established the gene order metA pepE zja-861::Tn5 malB. Duplications of the pepE locus showed a gene dosage effect on levels of peptidase E, suggesting that pepE is the structural gene for this enzyme. Mutations in pepE resulted in the loss of the ability to grow on Asp-Pro as a proline source but did not affect utilization of other dipeptides with N-terminal aspartic acid. Loss of peptidase E did not cause a detectable impairment in protein degradation. Two other peptidases present in cell extracts of mutants lacking peptidases N, A, B, D, P, Q, and E also hydrolyze many Asp-X dipeptides.
Full text
PDF

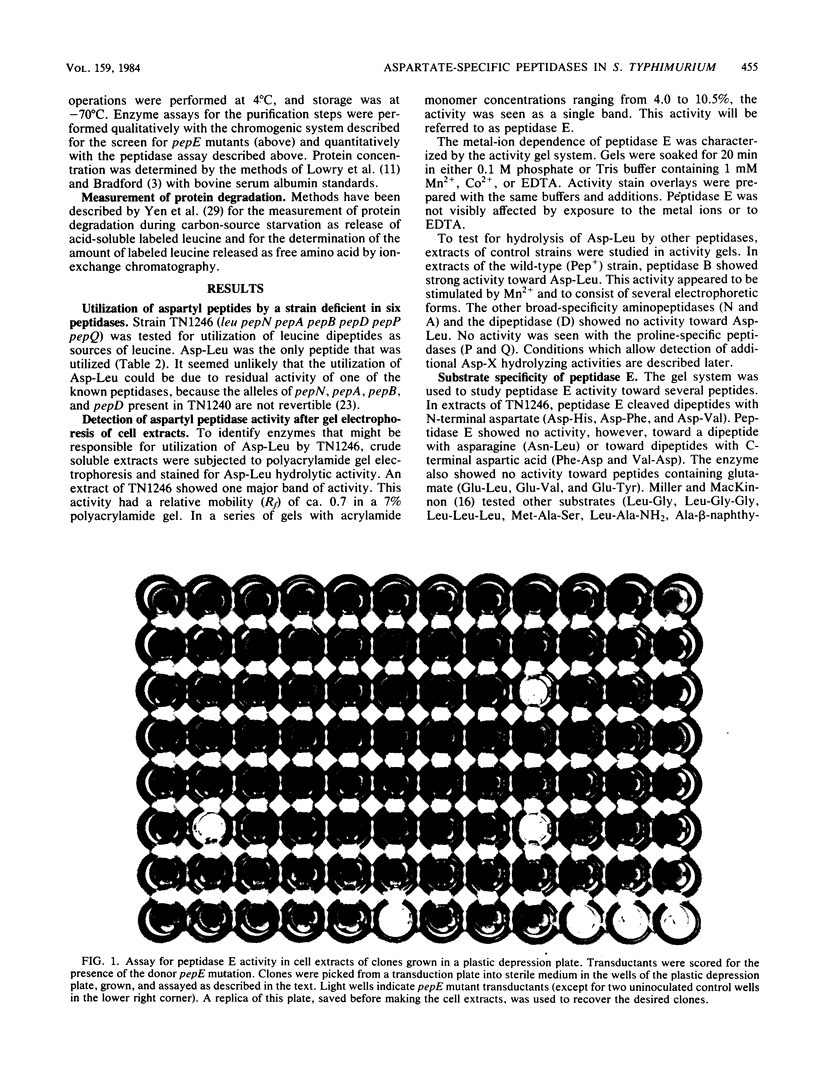

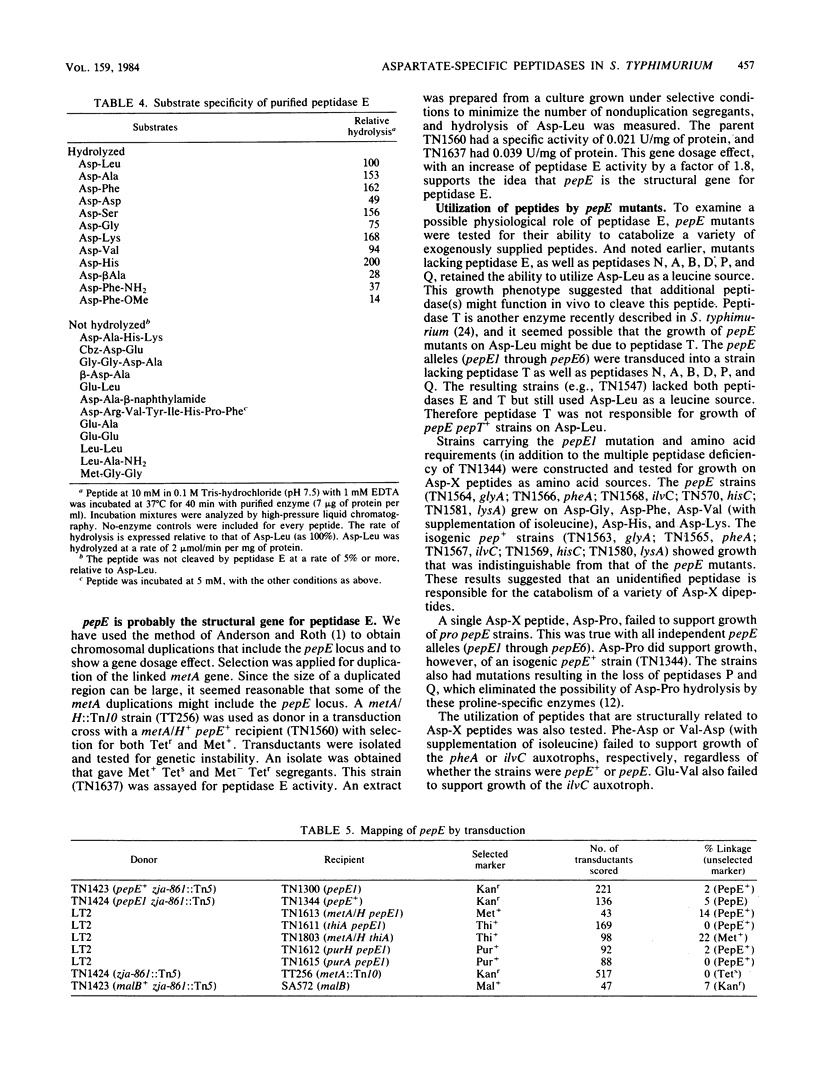


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. P., Roth J. R. Gene duplication in bacteria: alteration of gene dosage by sister-chromosome exchanges. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1083–1087. doi: 10.1101/sqb.1979.043.01.120. [DOI] [PubMed] [Google Scholar]
- Benajiba A., Maroux S. Purification and characterization of an aminopeptidase A from hog intestinal brush-border membrane. Eur J Biochem. 1980 Jun;107(2):381–388. doi: 10.1111/j.1432-1033.1980.tb06040.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cheung H. S., Cushman D. W. A soluble aspartate aminopeptidase from dog kidney. Biochim Biophys Acta. 1971 Jul 21;242(1):190–193. doi: 10.1016/0005-2744(71)90098-2. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley E. E. Purification and properties of a beta-aspartyl peptidase from Escherichia coli. J Biol Chem. 1968 Nov 10;243(21):5748–5752. [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsh M., Dembinski D. R., Hartman P. E., Miller C. G. Salmonella typhimurium peptidase active on carnosine. J Bacteriol. 1978 May;134(2):361–374. doi: 10.1128/jb.134.2.361-374.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McHugh G. L., Miller C. G. Isolation and characterization of proline peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):364–371. doi: 10.1128/jb.120.1.364-371.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Green L. Degradation of abnormal proteins in peptidase-deficient mutants of Salmonella typhimurium. J Bacteriol. 1981 Sep;147(3):925–930. doi: 10.1128/jb.147.3.925-930.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Green L. Degradation of proline peptides in peptidase-deficient strains of Salmonella typhimurium. J Bacteriol. 1983 Jan;153(1):350–356. doi: 10.1128/jb.153.1.350-356.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):355–363. doi: 10.1128/jb.120.1.355-363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G. Peptidases and proteases of Escherichia coli and Salmonella typhimurium. Annu Rev Microbiol. 1975;29:485–504. doi: 10.1146/annurev.mi.29.100175.002413. [DOI] [PubMed] [Google Scholar]
- Nagatsu I., Nagatsu T., Yamamoto T., Glenner G. G., Mehl J. W. Purification of aminopeptidase A in human serum and degradation of angiotensin II by the purified enzyme. Biochim Biophys Acta. 1970 Feb 11;198(2):255–270. doi: 10.1016/0005-2744(70)90058-6. [DOI] [PubMed] [Google Scholar]
- Patterson E. K., Gatmaitan J. S., Hayman S. Substrate specificity and pH dependence of dipeptidases purified from Escherichia coli B and from mouse ascites tumor cells. Biochemistry. 1973 Sep 11;12(19):3701–3709. doi: 10.1021/bi00743a020. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Ross H., Ziegler L., Mäkelä P. H. F + , Hfr, and F' strains of Salmonella typhimurium and Salmonella abony. Bacteriol Rev. 1972 Dec;36(4):608–637. doi: 10.1128/br.36.4.608-637.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Carter T. H., Miller C. G. Overproduction of Salmonella typhimurium peptidase T. J Bacteriol. 1983 Nov;156(2):743–751. doi: 10.1128/jb.156.2.743-751.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Miller C. G. Isolation and characterization Salmonella typhimurium mutants lacking a tripeptidase (peptidase T). J Bacteriol. 1983 May;154(2):763–771. doi: 10.1128/jb.154.2.763-771.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe H., Kojima F., Aoyagi T., Umezawa H. Purification by affinity chromatography using amastatin and properties of aminopeptidase A from pig kidney. Biochim Biophys Acta. 1980 Jun 13;613(2):459–468. doi: 10.1016/0005-2744(80)90100-x. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vimr E. R., Green L., Miller C. G. Oligopeptidase-deficient mutants of Salmonella typhimurium. J Bacteriol. 1983 Mar;153(3):1259–1265. doi: 10.1128/jb.153.3.1259-1265.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Miller C. G. Dipeptidyl carboxypeptidase-deficient mutants of Salmonella typhimurium. J Bacteriol. 1983 Mar;153(3):1252–1258. doi: 10.1128/jb.153.3.1252-1258.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C., Green L., Miller C. G. Degradation of intracellular protein in Salmonella typhimurium peptidase mutants. J Mol Biol. 1980 Oct 15;143(1):21–33. doi: 10.1016/0022-2836(80)90122-9. [DOI] [PubMed] [Google Scholar]
- Yen C., Green L., Miller C. G. Peptide accumulation during growth of peptidase deficient mutants. J Mol Biol. 1980 Oct 15;143(1):35–48. doi: 10.1016/0022-2836(80)90123-0. [DOI] [PubMed] [Google Scholar]



