Abstract
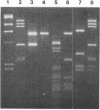
Small plasmids which replicate in both Escherichia coli and Clostridium perfringens were made by recombining E. coli plasmid pBR322 with three different small (less than 4 kilobases) plasmids native to C. perfringens. Subsequently, two homologous, though distinct, tetracycline resistance determinants (tet) from other C. perfringens plasmids were cloned into them. Both tet systems made E. coli resistant to at least 5 micrograms of tetracycline per ml when resident on the shuttle plasmids. The shuttle vectors have been used to transform L-phase variants and autoplasts of C. perfringens. In the latter case, the intact transforming plasmid could be isolated from walled cells after cell wall regeneration. Reciprocal transformation experiments in which plasmid DNAs derived from E. coli or C. perfringens were used suggest that restriction barriers exist between these two organisms. The plasmids contain restriction enzyme recognition sites in locations which are useful for cloning experiments.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Heefner D. L., Squires C. H., Evans R. J., Kopp B. J., Yarus M. J. Transformation of Clostridium perfringens. J Bacteriol. 1984 Aug;159(2):460–464. doi: 10.1128/jb.159.2.460-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A. W., Krell P. J., Mahony D. E. Plasmid detection in a bacteriocinogenic strain of Clostridium perfringens. Can J Microbiol. 1980 Aug;26(8):1018–1022. doi: 10.1139/m80-173. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rood J. I., Maher E. A., Somers E. B., Campos E., Duncan C. L. Isolation and characterization of multiply antibiotic-resistant Clostridum perfringens strains from porcine feces. Antimicrob Agents Chemother. 1978 May;13(5):871–880. doi: 10.1128/aac.13.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood J. I., Scott V. N., Duncan C. L. Identification of a transferable tetracycline resistance plasmid (pCW3) from Clostridium perfringens. Plasmid. 1978 Sep;1(4):563–570. doi: 10.1016/0147-619x(78)90013-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]