Abstract
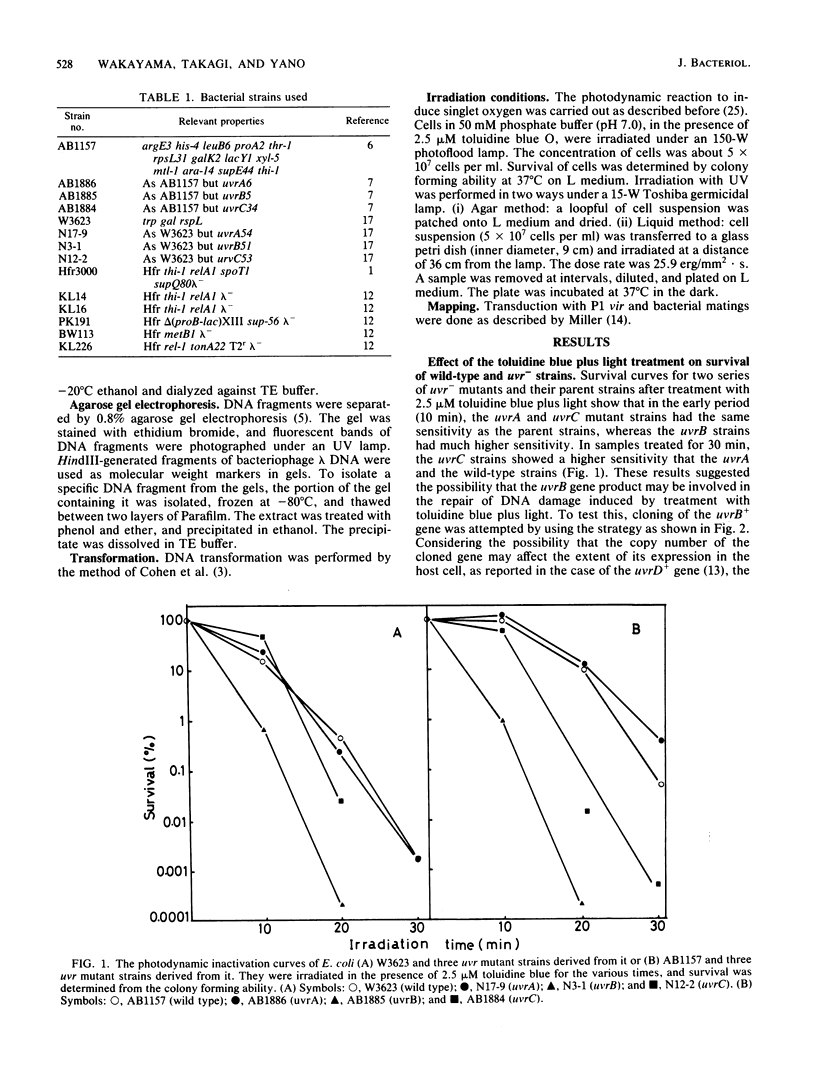
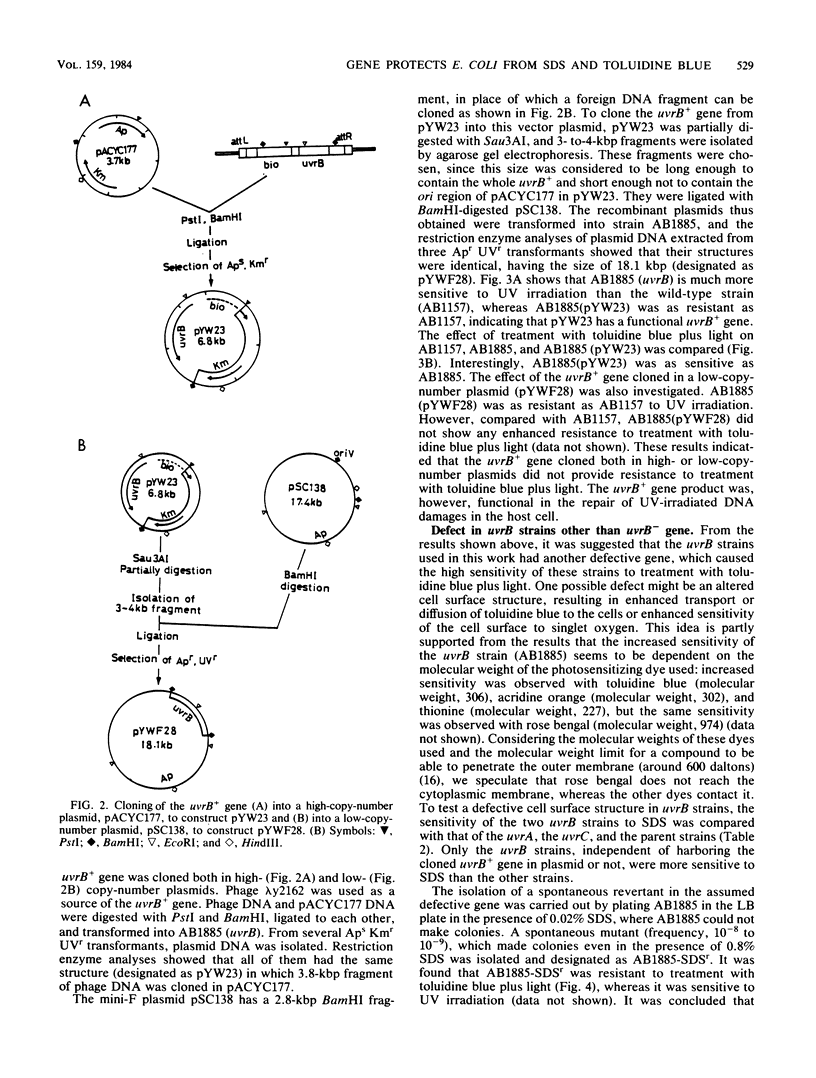
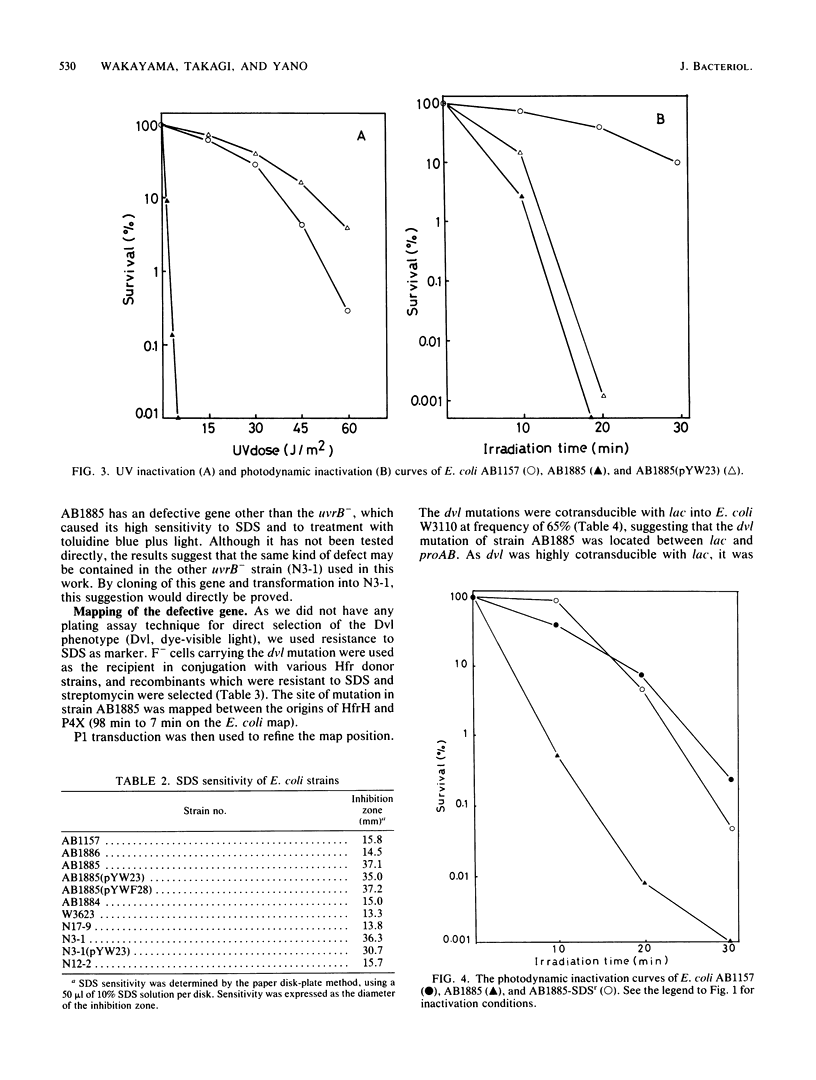
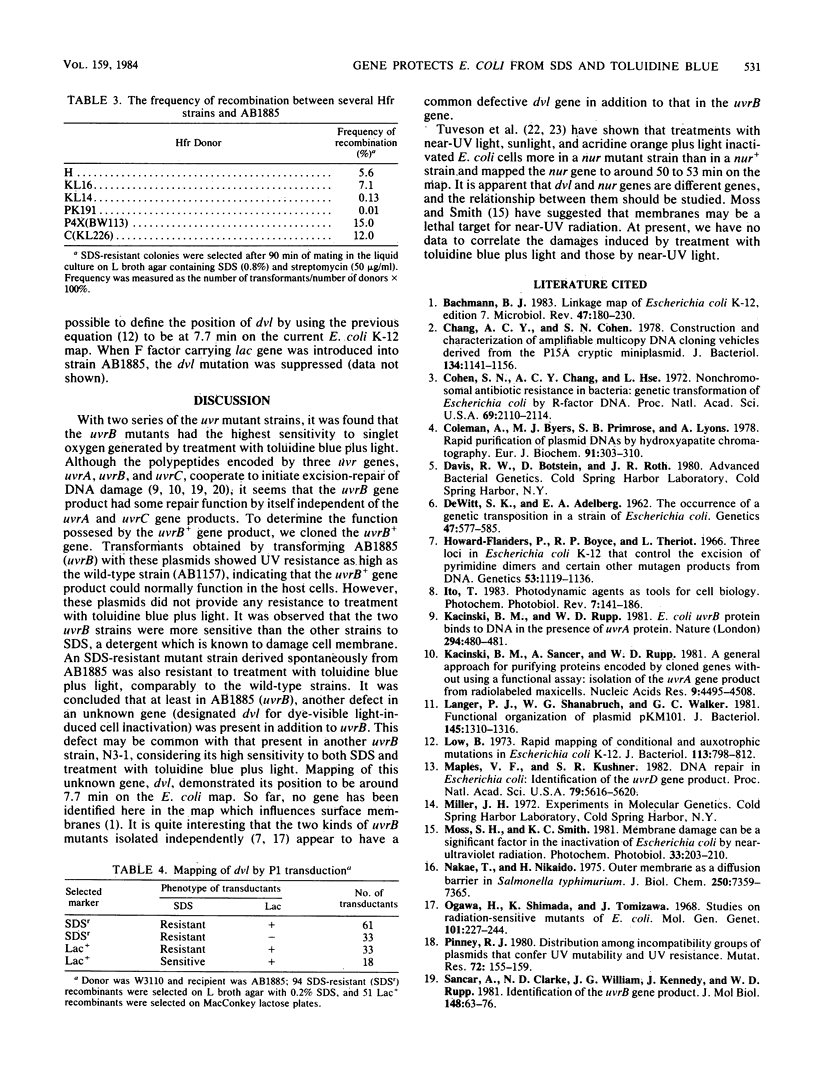
Escherichia coli cells were killed by visible light irradiation in the presence of the photosensitizing dye, toluidine blue. Two uvrB mutant strains of E. coli K-12 (AB1885 and N3-1) were much more sensitive than the isogenic uvrA and uvrC strains to treatment with toluidine blue plus light, suggesting that the uvrB+ gene product was involved in repair of DNA damage induced by the treatment. The uvrB+ gene cloned in a high- or low-copy-number plasmid was transformed into the uvrB strain (AB1885). Although all the transformants showed the same resistance as its wild-type strain (AB1157) to UV irradiation, they were as sensitive as AB1885 was to treatment with toluidine blue plus light. The two uvrB strains were more sensitive to sodium dodecyl sulfate than the other strains, suggesting that these strains had a defect in the cell surface. A sodium dodecyl sulfate-resistant revertant obtained from AB1885 was more resistant than AB1885 was to treatment with toluidine blue plus light. The two uvrB strains (AB1885 and N3-1) appear to have a defective gene (tentatively called dvl) different from uvrB. Its map position was around 7 min on the E. coli map.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman A., Byers M. J., Primrose S. B., Lyons A. Rapid purification of plasmid DNAs by hydroxyapatite chromatography. Eur J Biochem. 1978 Nov 2;91(1):303–310. doi: 10.1111/j.1432-1033.1978.tb20966.x. [DOI] [PubMed] [Google Scholar]
- Dewitt S K, Adelberg E A. The Occurrence of a Genetic Transposition in a Strain of Escherichia Coli. Genetics. 1962 May;47(5):577–585. doi: 10.1093/genetics/47.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacinski B. M., Rupp W. D. E. coli uvrB protein binds to DNA in the presence of uvrA protein. Nature. 1981 Dec 3;294(5840):480–481. doi: 10.1038/294480a0. [DOI] [PubMed] [Google Scholar]
- Kacinski B. M., Sancar A., Rupp W. D. A general approach for purifying proteins encoded by cloned genes without using a functional assay: isolation of the uvrA gene product from radiolabeled maxicells. Nucleic Acids Res. 1981 Sep 25;9(18):4495–4508. doi: 10.1093/nar/9.18.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. J., Shanabruch W. G., Walker G. C. Functional organization of plasmid pKM101. J Bacteriol. 1981 Mar;145(3):1310–1316. doi: 10.1128/jb.145.3.1310-1316.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maples V. F., Kushner S. R. DNA repair in Escherichia coli: identification of the uvrD gene product. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5616–5620. doi: 10.1073/pnas.79.18.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss S. H., Smith K. C. Membrane damage can be a significant factor in the inactivation of Escherichia coli by near-ultraviolet radiation. Photochem Photobiol. 1981 Feb;33(2):203–210. doi: 10.1111/j.1751-1097.1981.tb05325.x. [DOI] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- Pinney R. J. Distribution among incompatibility groups of plasmids that confer UV mutability and UV resistance. Mutat Res. 1980 Aug;72(1):155–159. doi: 10.1016/0027-5107(80)90232-8. [DOI] [PubMed] [Google Scholar]
- Sancar A., Clarke N. D., Griswold J., Kennedy W. J., Rupp W. D. Identification of the uvrB gene product. J Mol Biol. 1981 May 5;148(1):63–76. doi: 10.1016/0022-2836(81)90235-7. [DOI] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuveson R. W., Jonas R. B. Genetic control of near-UV (300-400 NM) sensitivity independent of the recA gene in strains of Escherichia coli K12. Photochem Photobiol. 1979 Dec;30(6):667–676. doi: 10.1111/j.1751-1097.1979.tb07197.x. [DOI] [PubMed] [Google Scholar]
- Wagner S., Taylor W. D., Keith A., Snipes W. Effects of acridine plus near ultraviolet light on Escherichia coli membranes and DNA in vivo. Photochem Photobiol. 1980 Dec;32(6):771–779. doi: 10.1111/j.1751-1097.1980.tb04054.x. [DOI] [PubMed] [Google Scholar]
- Wakayama Y., Takagi M., Yano K. Photosensitized inactivation of E. coli cells in toluidine blue-light system. Photochem Photobiol. 1980 Nov;32(5):601–605. doi: 10.1111/j.1751-1097.1980.tb04028.x. [DOI] [PubMed] [Google Scholar]
- Yano K., Homma S., Takagi M. Degradative plasmid TOL provided singlet oxygen resistance for Pseudomonas strains. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1245–1250. doi: 10.1016/0006-291x(81)90753-1. [DOI] [PubMed] [Google Scholar]
- Yonei S., Furui H. Lethal and mutagenic effects of malondialdehyde, a decomposition product of peroxidized lipids, on Escherichia coli with different DNA-repair capacities. Mutat Res. 1981 Jan;88(1):23–32. doi: 10.1016/0165-1218(81)90086-0. [DOI] [PubMed] [Google Scholar]



