Abstract
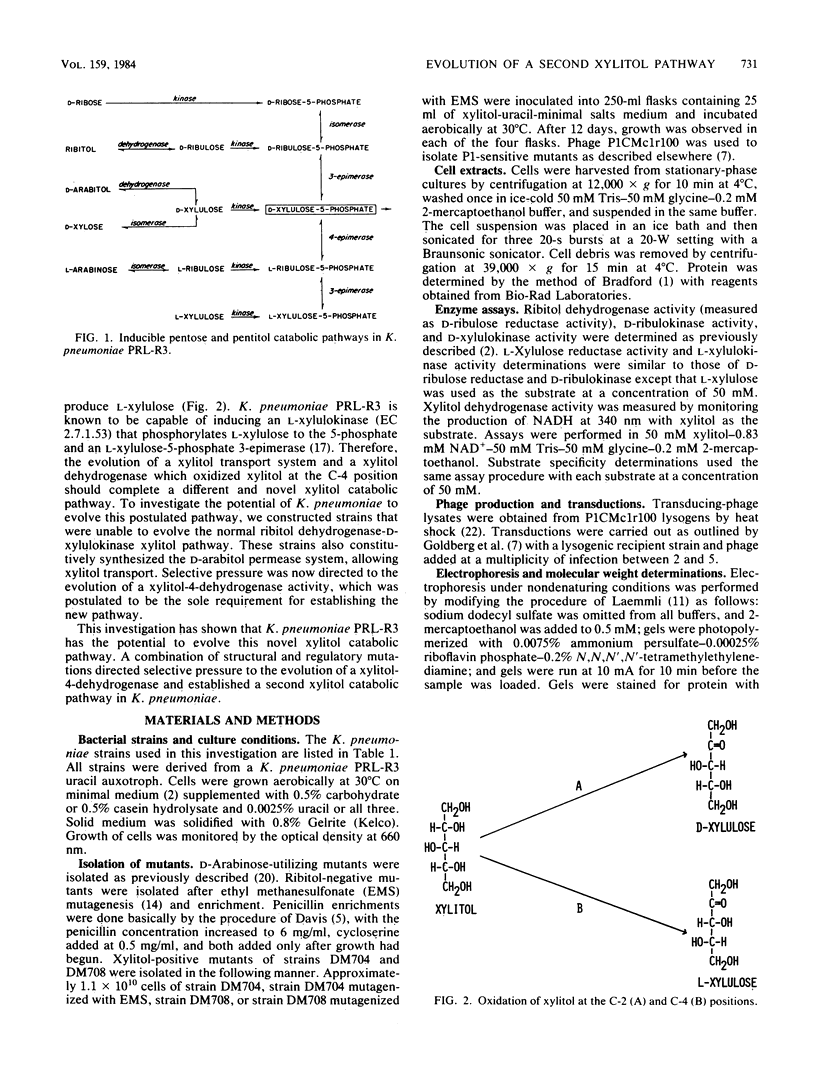
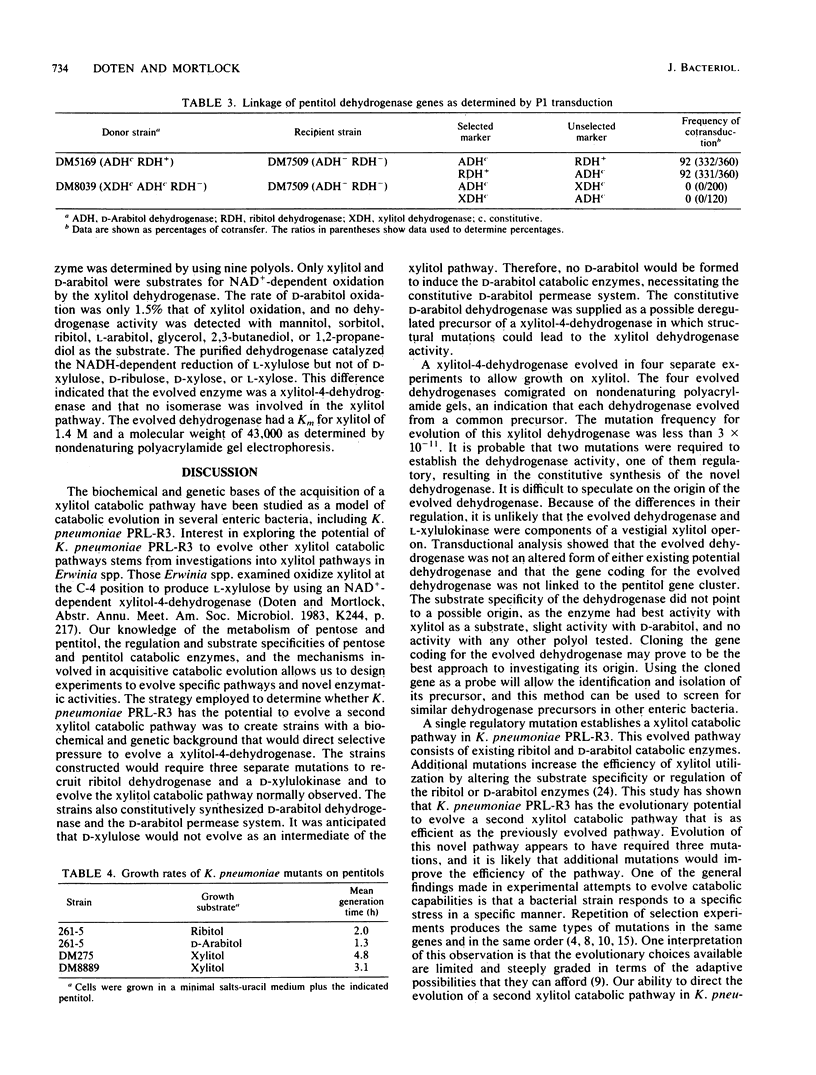
Klebsiella pneumoniae PRL-R3 has inducible catabolic pathways for the degradation of ribitol and D-arabitol but cannot utilize xylitol as a growth substrate. A mutation in the rbtB regulatory gene of the ribitol operon permits the constitutive synthesis of the ribitol catabolic enzymes and allows growth on xylitol. The evolved xylitol catabolic pathway consists of an induced D-arabitol permease system that also transports xylitol, a constitutively synthesized ribitol dehydrogenase that oxidizes xylitol at the C-2 position to produce D-xylulose, and an induced D-xylulokinase from either the D-arabitol or D-xylose catabolic pathway. To investigate the potential of K. pneumoniae to evolve a different xylitol catabolic pathway, strains were constructed which were unable to synthesize ribitol dehydrogenase or either type of D-xylulokinase but constitutively synthesized the D-arabitol permease system. These strains had an inducible L-xylulokinase; therefore, the evolution of an enzyme which oxidized xylitol at the C-4 position to L-xylulose would establish a new xylitol catabolic pathway. Four independent xylitol-utilizing mutants were isolated, each of which had evolved a xylitol-4-dehydrogenase activity. The four dehydrogenases appeared to be identical because they comigrated during nondenaturing polyacrylamide gel electrophoresis. This novel xylitol dehydrogenase was constitutively synthesized, whereas L-xylulokinase remained inducible. Transductional analysis showed that the evolved dehydrogenase was not an altered ribitol or D-arabitol dehydrogenase and that the evolved dehydrogenase structural gene was not linked to the pentitol gene cluster. This evolved dehydrogenase had the highest activity with xylitol as a substrate, a Km for xylitol of 1.4 M, and a molecular weight of 43,000.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Charnetzky W. T., Mortlock R. P. Close genetic linkage of the determinants of the ribitol and D-arabitol catabolic pathways in Klebsiella aerogenes. J Bacteriol. 1974 Jul;119(1):176–182. doi: 10.1128/jb.119.1.176-182.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnetzky W. T., Mortlock R. P. Ribitol catabolic pathway in Klebsiella aerogenes. J Bacteriol. 1974 Jul;119(1):162–169. doi: 10.1128/jb.119.1.162-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Bender R. A., Streicher S. L. Direct selection for P1-sensitive mutants of enteric bacteria. J Bacteriol. 1974 Jun;118(3):810–814. doi: 10.1128/jb.118.3.810-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansche P. E. Gene duplication as a mechanism of genetic adaptation in Saccharomyces cerevisiae. Genetics. 1975 Apr;79(4):661–674. doi: 10.1093/genetics/79.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A. Enzyme recruitment in evolution of new function. Annu Rev Microbiol. 1976;30:409–425. doi: 10.1146/annurev.mi.30.100176.002205. [DOI] [PubMed] [Google Scholar]
- LERNER S. A., WU T. T., LIN E. C. EVOLUTION OF A CATABOLIC PATHWAY IN BACTERIA. Science. 1964 Dec 4;146(3649):1313–1315. doi: 10.1126/science.146.3649.1313. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- London J., Chace N. M. New pathway for the metabolism of pentitols. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4296–4300. doi: 10.1073/pnas.74.10.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
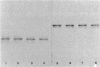
- MORTLOCK R. P., WOOD W. A. METABOLISM OF PENTOSES AND PENTITOLS BY AEROBACTER AEROGENES. I. DEMONSTRATION OF PENTOSE ISOMERASE, PENTULOKINASE, AND PENTITOL DEHYDROGENASE ENZYME FAMILIES. J Bacteriol. 1964 Oct;88:838–844. doi: 10.1128/jb.88.4.838-844.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortlock R. P., Fossitt D. D., Wood W. A. A basis for utlization of unnatural pentoses and pentitols by Aerobacter aerogenes. Proc Natl Acad Sci U S A. 1965 Aug;54(2):572–579. doi: 10.1073/pnas.54.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Patterson R. A., Hartley B. S. Purification and properties of Klebsiella aerogenes D-arabitol dehydrogenase. Biochem J. 1979 Oct 1;183(1):31–42. doi: 10.1042/bj1830031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver E. J., Bisson T. M., LeBlanc D. J., Mortlock R. P. D-Ribulose production by a mutant of Aerobacter aerogens. Anal Biochem. 1969 Feb;27(2):300–305. doi: 10.1016/0003-2697(69)90036-0. [DOI] [PubMed] [Google Scholar]
- Oliver E. J., Mortlock R. P. Growth of Aerobacter aerogenes on D-arabinose: origin of the enzyme activities. J Bacteriol. 1971 Oct;108(1):287–292. doi: 10.1128/jb.108.1.287-292.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M. Xylitol and D-arabitol toxicities due to derepressed fructose, galactitol, and sorbitol phosphotransferases of Escherichia coli. J Bacteriol. 1977 Oct;132(1):166–173. doi: 10.1128/jb.132.1.166-173.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Scangos G. A., Reiner A. M. Ribitol and D-arabitol catabolism in Escherichia coli. J Bacteriol. 1978 May;134(2):492–500. doi: 10.1128/jb.134.2.492-500.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T., Lin E. C., Tanaka S. Mutants of Aerobacter aerogenes capable of utilizing xylitol as a novel carbon. J Bacteriol. 1968 Aug;96(2):447–456. doi: 10.1128/jb.96.2.447-456.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]