Abstract
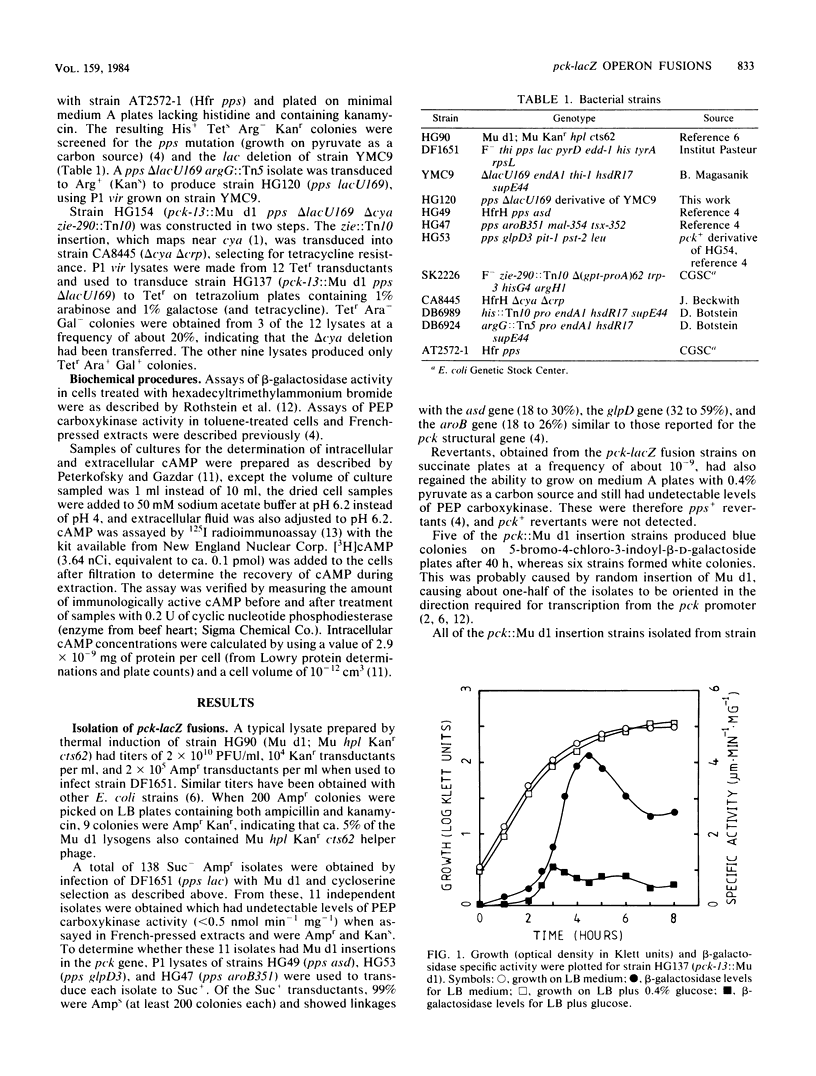
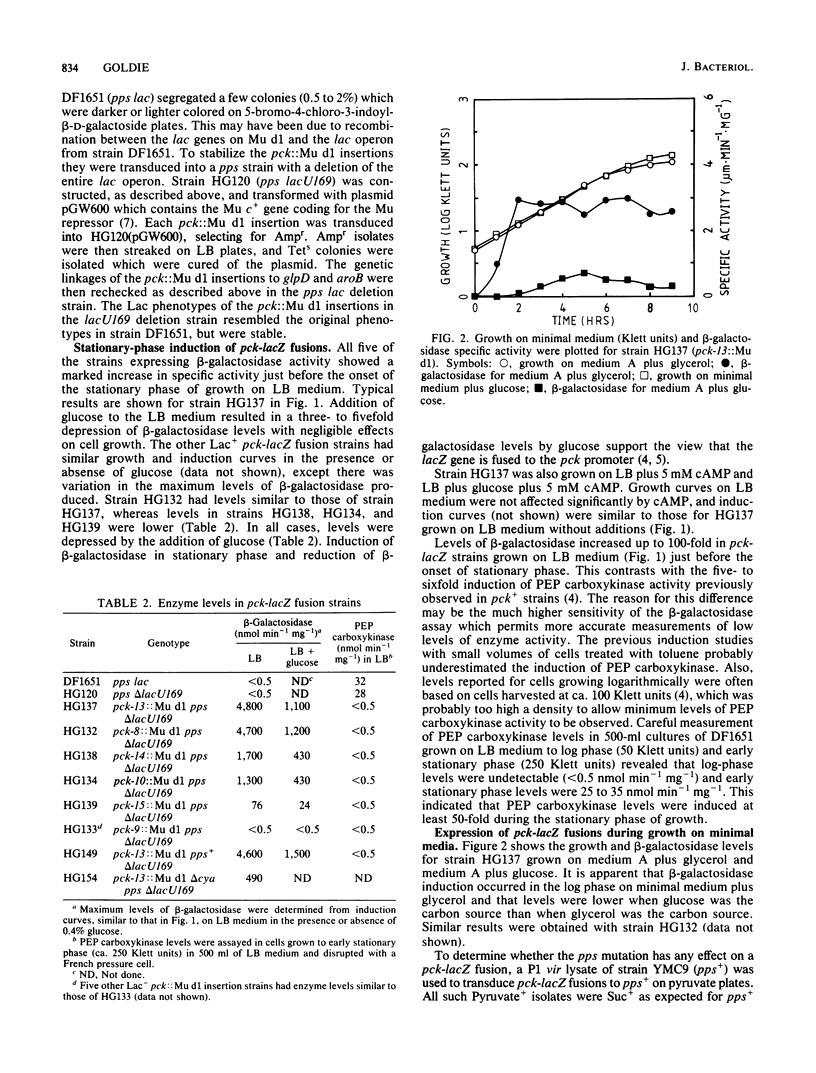
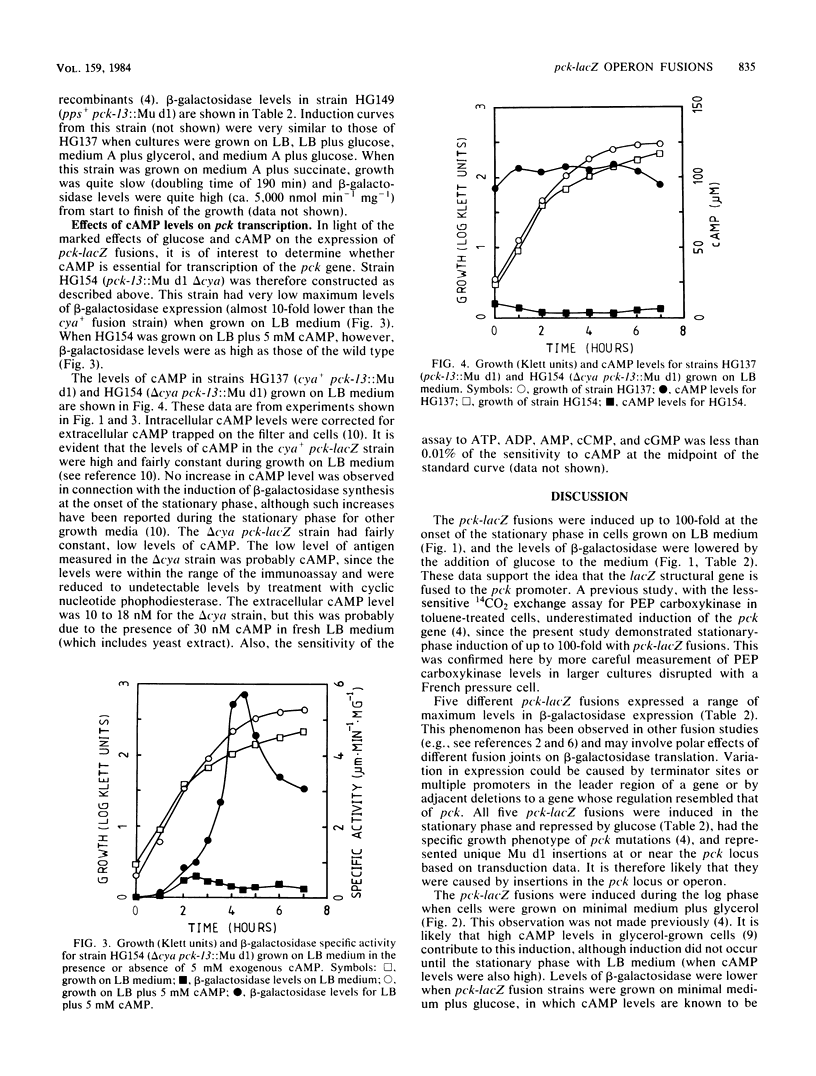
Mutants of Escherichia coli containing genetic fusions of lacZ to the pck (phosphoenolpyruvate carboxykinase) locus were isolated by using Mu d(lacZ Ampr) bacteriophage. Synthesis of beta-galactosidase in these strains is regulated by cyclic AMP and glucose (catabolite repression). Synthesis of beta-galactosidase by pck-lacZ fusions was induced in log-phase cells growing on gluconeogenic media, was repressed by glucose, and was also induced up to 100-fold at the onset of stationary phase in LB medium. This stationary-phase induction required cyclic AMP and some other unknown regulatory signal.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie A. H., Narindrasorasak S., Sanwal B. D. An unusual type of regulation of malate oxidase synthesis in Escherichia coli. Biochem Biophys Res Commun. 1978 Jul 28;83(2):421–426. doi: 10.1016/0006-291x(78)91007-0. [DOI] [PubMed] [Google Scholar]
- Goldie A. H., Sanwal B. D. Genetic and physiological characterization of Escherichia coli mutants deficient in phosphoenolpyruvate carboxykinase activity. J Bacteriol. 1980 Mar;141(3):1115–1121. doi: 10.1128/jb.141.3.1115-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie A. H., Sanwal B. D. Temperature-sensitive mutation affecting synthesis of phosphoenolpyruvate carboxykinase in Escherichia coli. J Bacteriol. 1981 Nov;148(2):720–723. doi: 10.1128/jb.148.2.720-723.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie H., Magasanik B. Effects of glnL and other regulatory loci on regulation of transcription of glnA-lacZ fusions in Klebsiella aerogenes. J Bacteriol. 1982 Apr;150(1):231–238. doi: 10.1128/jb.150.1.231-238.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. H., Walker G. C. Mud(Ap, lac)-generated fusions in studies of gene expression. Methods Enzymol. 1983;100:501–509. doi: 10.1016/0076-6879(83)00075-0. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Glucose inhibition of adenylate cyclase in intact cells of Escherichia coli B. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2324–2328. doi: 10.1073/pnas.71.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein D. M., Pahel G., Tyler B., Magasanik B. Regulation of expression from the glnA promoter of Escherichia coli in the absence of glutamine synthetase. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7372–7376. doi: 10.1073/pnas.77.12.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John A. C., Conklin K., Rosenthal E., Goldberg A. L. Further evidence for the involvement of charged tRNA and guanosine tetraphosphate in the control of protein degradation in Escherichia coli. J Biol Chem. 1978 Jun 10;253(11):3945–3951. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Steiner K. E., Preiss J. Biosynthesis of bacterial glycogen: genetic and allosteric regulation of glycogen biosynthesis in Salmonella typhimurium LT-2. J Bacteriol. 1977 Jan;129(1):246–253. doi: 10.1128/jb.129.1.246-253.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Kodaira R., Neidhardt F. C. Regulation of lac operon expression: reappraisal of the theory of catabolite repression. J Bacteriol. 1978 Dec;136(3):947–954. doi: 10.1128/jb.136.3.947-954.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



