Abstract
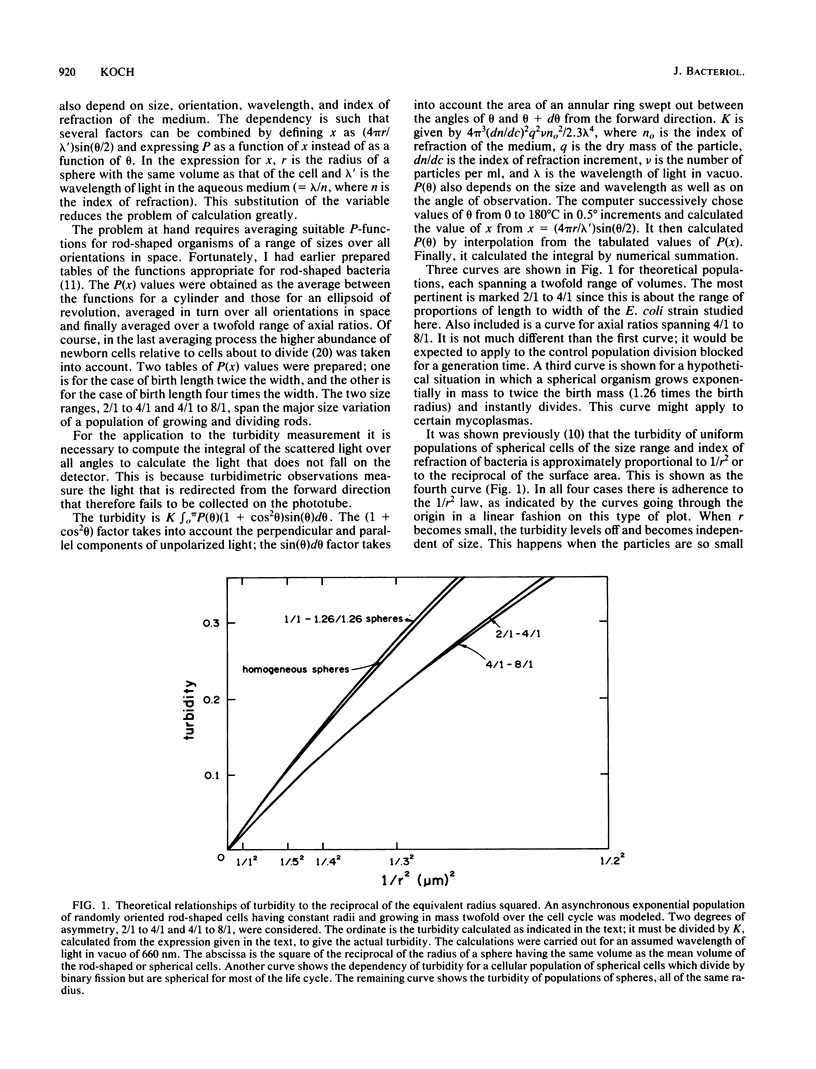
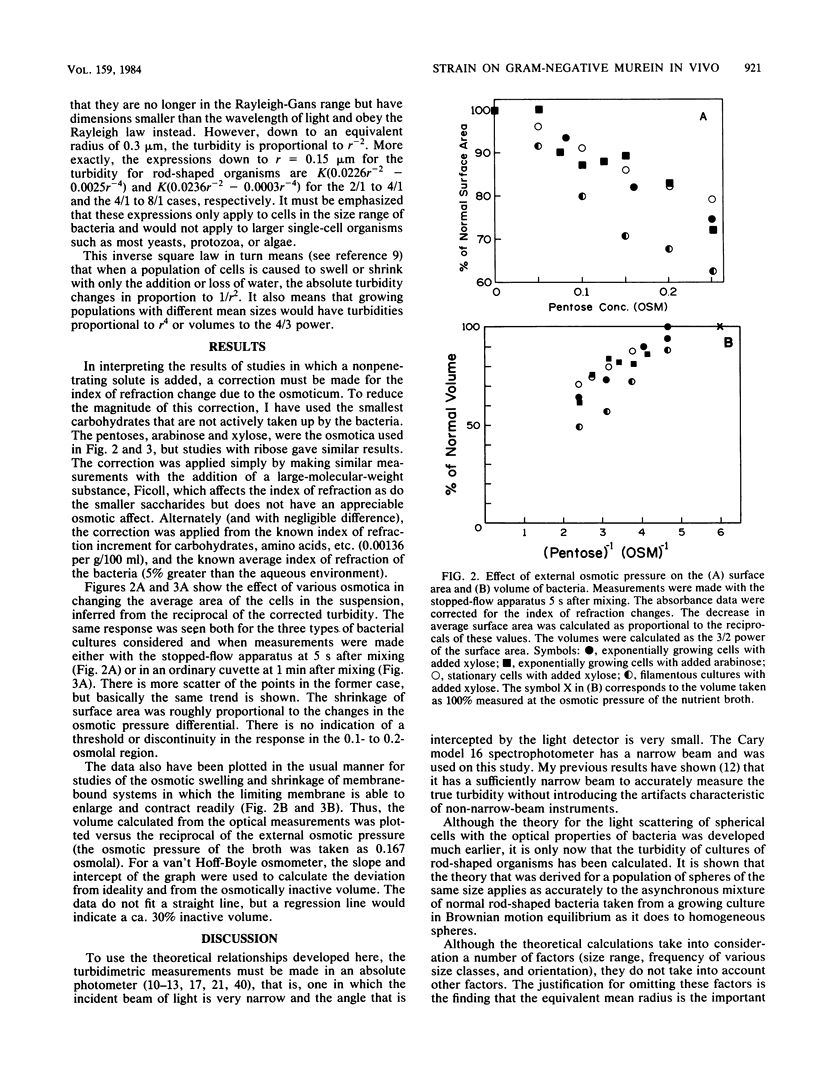
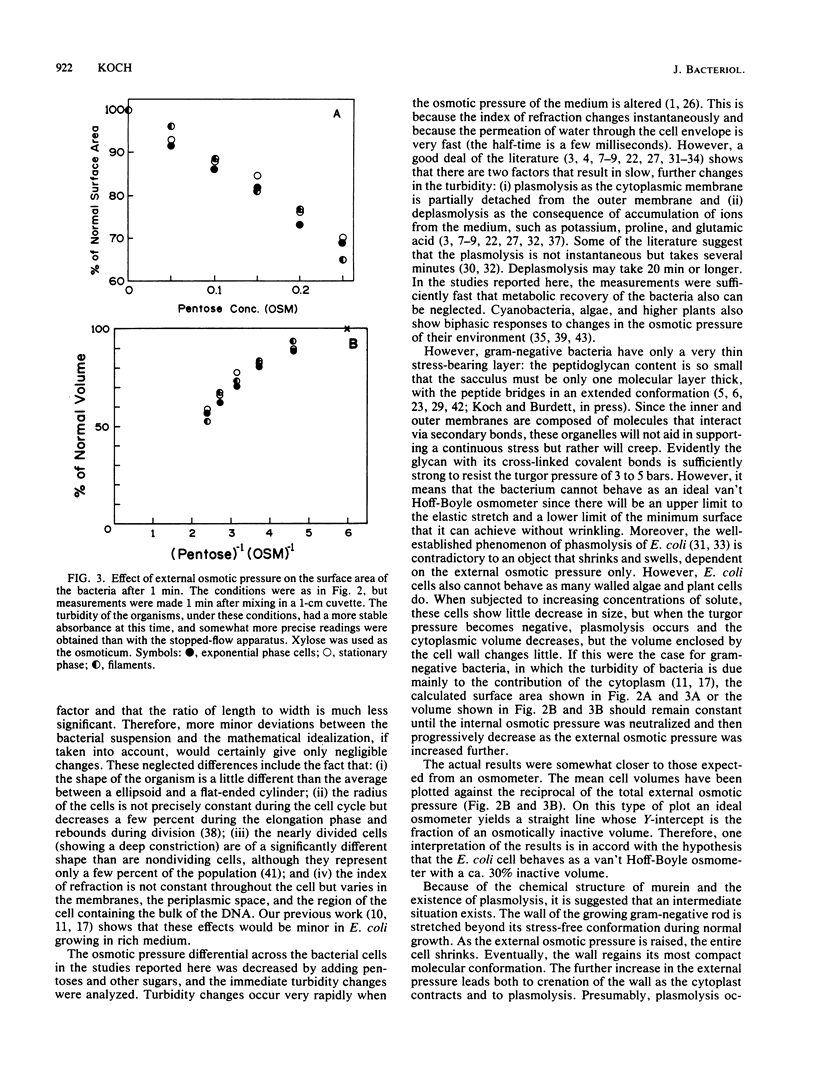
The immediate response of growing Escherichia coli to changing external osmotic pressure was studied with stopped-flow turbidimetric measurements with a narrow-beam spectrophotometer. It is shown theoretically that in such a photometer rod-shaped bacteria have an apparent absorbance which is proportional to the inverse of the surface area. The apparent optical density, corrected for effects of alteration of the index of refraction of the medium, increased continuously as the external osmotic pressure was raised. Because of the short time scale of the measurements, the turbidity increases could result either from shrinkage of the cells or from plasmolysis, or both, but not from growth or metabolic adaptation. With low concentrations of pentose such that the external osmotic pressure was not greater than that inside the cells, plasmolysis would not occur and, consequently, only shrinkage of the previously stretched sacculus remains to account for the observed optical effects. Taking the osmotic pressure of the growing cells as 5 atmospheres (506 kPa), the turbidity changes correspond to the murein fabric having been stretched 20% beyond its unstressed equilibrium area during growth under the conditions used.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVI-DOR Y., KUCZYNSKI M., SCHATZBERG G., MAGER J. Turbidity changes in bacterial suspensions: kinetics and relation to metabolic state. J Gen Microbiol. 1956 Feb;14(1):76–83. doi: 10.1099/00221287-14-1-76. [DOI] [PubMed] [Google Scholar]
- Alemohammad M. M., Knowles C. J. Osmotically induced volume and turbidity changes of Escherichia coli due to salts, sucrose and glycerol, with particular reference to the rapid permeation of glycerol into the cell. J Gen Microbiol. 1974 May;82(1):125–142. doi: 10.1099/00221287-82-1-125. [DOI] [PubMed] [Google Scholar]
- Allen J. S., Filip C. C., Gustafson R. A., Allen R. G., Walker J. R. Regulation of bacterial cell division: genetic and phenotypic analysis of temperature-sensitive, multinucleate, filament-forming mutants of Escherichia. J Bacteriol. 1974 Mar;117(3):978–986. doi: 10.1128/jb.117.3.978-986.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVELL C. R., PACKER L., HELGERSON R. PERMEABILITY OF ESCHERICHIA COLI TO ORGANIC COMPOUNDS AND INORGANIC SALTS MEASURED BY LIGHT-SCATTERING. Biochim Biophys Acta. 1963 Sep 24;75:257–266. doi: 10.1016/0006-3002(63)90604-8. [DOI] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH A. L., SCHAECHTER M. A model for statistics of the cell division process. J Gen Microbiol. 1962 Nov;29:435–454. doi: 10.1099/00221287-29-3-435. [DOI] [PubMed] [Google Scholar]
- KOCH A. L. Some calculations on the turbidity of mitochondria and bacteria. Biochim Biophys Acta. 1961 Aug 19;51:429–441. doi: 10.1016/0006-3002(61)90599-6. [DOI] [PubMed] [Google Scholar]
- Knowles C. J. Salt induces changes of turbidity and volume of E. coli. Nat New Biol. 1971 Feb 3;229(5):154–155. doi: 10.1038/newbio229154a0. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Ehrenfeld E. Th size and shape of bacteria by light scattering measurements. Biochim Biophys Acta. 1968 Sep 3;165(2):262–273. doi: 10.1016/0304-4165(68)90054-8. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Higgins M. L., Doyle R. J. Surface tension-like forces determine bacterial shapes: Streptococcus faecium. J Gen Microbiol. 1981 Mar;123(1):151–161. doi: 10.1099/00221287-123-1-151. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Higgins M. L., Doyle R. J. The role of surface stress in the morphology of microbes. J Gen Microbiol. 1982 May;128(5):927–945. doi: 10.1099/00221287-128-5-927. [DOI] [PubMed] [Google Scholar]
- Koch A. L. On the growth and form of Escherichia coli. J Gen Microbiol. 1982 Nov;128(11):2527–2539. doi: 10.1099/00221287-128-11-2527. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The surface stress theory of microbial morphogenesis. Adv Microb Physiol. 1983;24:301–366. doi: 10.1016/s0065-2911(08)60388-4. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Theory of the angular dependence of light scattered by bacteria and similar-sized biological objects. J Theor Biol. 1968 Jan;18(1):133–156. doi: 10.1016/0022-5193(68)90174-4. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal Biochem. 1970 Nov;38(1):252–259. doi: 10.1016/0003-2697(70)90174-0. [DOI] [PubMed] [Google Scholar]
- MAGER J., KUCZYNSKI M., SCHATZBERG G., AVI-DOR Y. Turbidity changes in bacterial suspensions in relation to osmotic pressure. J Gen Microbiol. 1956 Feb;14(1):69–75. doi: 10.1099/00221287-14-1-69. [DOI] [PubMed] [Google Scholar]
- Marquis R. E., Carstensen E. L. Electric conductivity and internal osmolality of intact bacterial cells. J Bacteriol. 1973 Mar;113(3):1198–1206. doi: 10.1128/jb.113.3.1198-1206.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E. Salt-induced contraction of bacterial cell walls. J Bacteriol. 1968 Mar;95(3):775–781. doi: 10.1128/jb.95.3.775-781.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matts T. C., Knowles C. J. Stopped-flow studies of salt-induced turbidity changes of Escherichia coli. Biochim Biophys Acta. 1971 Dec 3;249(2):583–587. doi: 10.1016/0005-2736(71)90134-9. [DOI] [PubMed] [Google Scholar]
- Matula T. I., MacLeod R. A. Mechanism of optical effects in suspensions of a marine pseudomonad. J Bacteriol. 1969 Oct;100(1):403–410. doi: 10.1128/jb.100.1.403-410.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldmixon E. H., Glauser S., Higgins M. L. Two proposed general configurations for bacterial cell wall peptidoglycans shown by space-filling molecular models. Biopolymers. 1974;13(10):2037–2060. doi: 10.1002/bip.1974.360131008. [DOI] [PubMed] [Google Scholar]
- Olijhoek A. J., Van Eden C. G., Trueba F. J., Pas E., Nanninga N. Plasmolysis during the division cycle of Escherichia coli. J Bacteriol. 1982 Oct;152(1):479–484. doi: 10.1128/jb.152.1.479-484.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou L. T., Marquis R. E. Electromechanical interactions in cell walls of gram-positive cocci. J Bacteriol. 1970 Jan;101(1):92–101. doi: 10.1128/jb.101.1.92-101.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheie P. O. Plasmolysis of Escherichia coli B-r with sucrose. J Bacteriol. 1969 May;98(2):335–340. doi: 10.1128/jb.98.2.335-340.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheie P. O., Rehberg R. Response of Escherichia coli B-r to high concentrations of sucrose in a nutrient medium. J Bacteriol. 1972 Jan;109(1):229–235. doi: 10.1128/jb.109.1.229-235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheie P. Osmotic pressure in Escherichia coli as rendered detectable by lysozyme attack. J Bacteriol. 1973 May;114(2):549–555. doi: 10.1128/jb.114.2.549-555.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970 Dec;64(2):171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]
- Trueba F. J., Woldringh C. L. Changes in cell diameter during the division cycle of Escherichia coli. J Bacteriol. 1980 Jun;142(3):869–878. doi: 10.1128/jb.142.3.869-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. H., Koch A. L. Constancy of growth on simple and complex media. J Bacteriol. 1978 Dec;136(3):969–975. doi: 10.1128/jb.136.3.969-975.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L., de Jong M. A., van den Berg W., Koppes L. Morphological analysis of the division cycle of two Escherichia coli substrains during slow growth. J Bacteriol. 1977 Jul;131(1):270–279. doi: 10.1128/jb.131.1.270-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Woldringh C. L., Mirelman D. Constant peptidoglycan density in the sacculus of Escherichia coli B/r growing at different rates. FEBS Lett. 1979 Feb 1;98(1):29–32. doi: 10.1016/0014-5793(79)80144-1. [DOI] [PubMed] [Google Scholar]


