Abstract
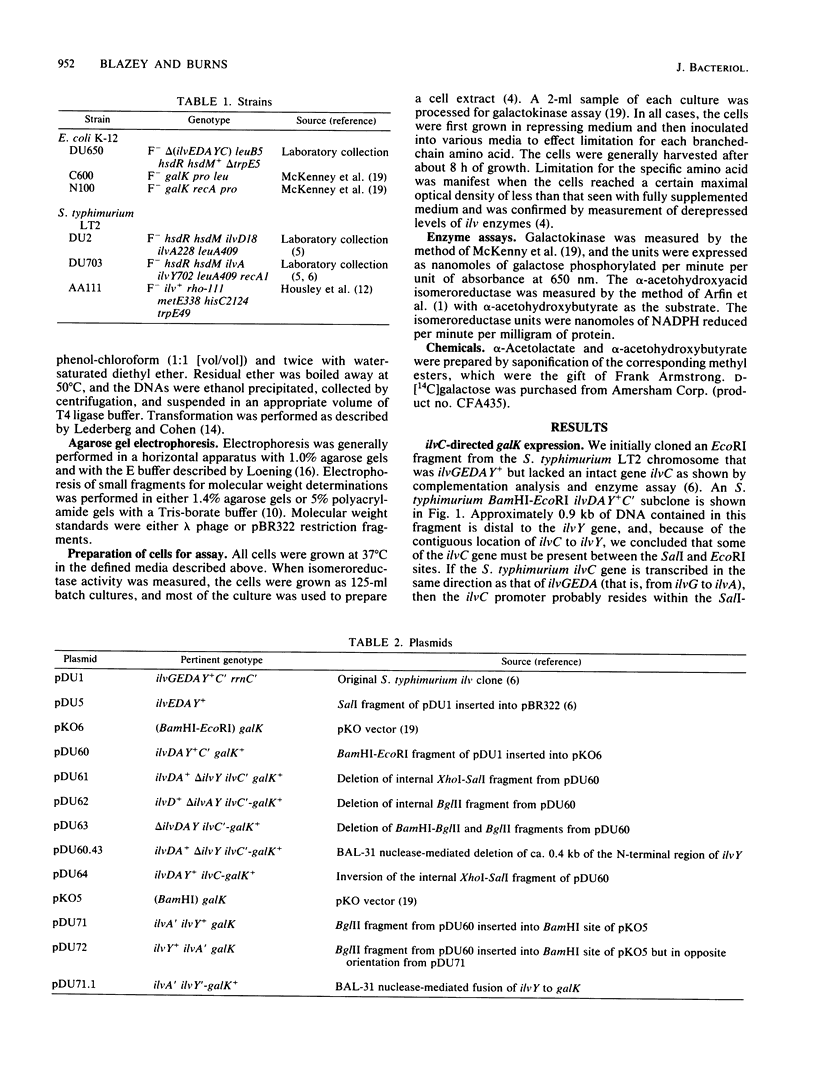
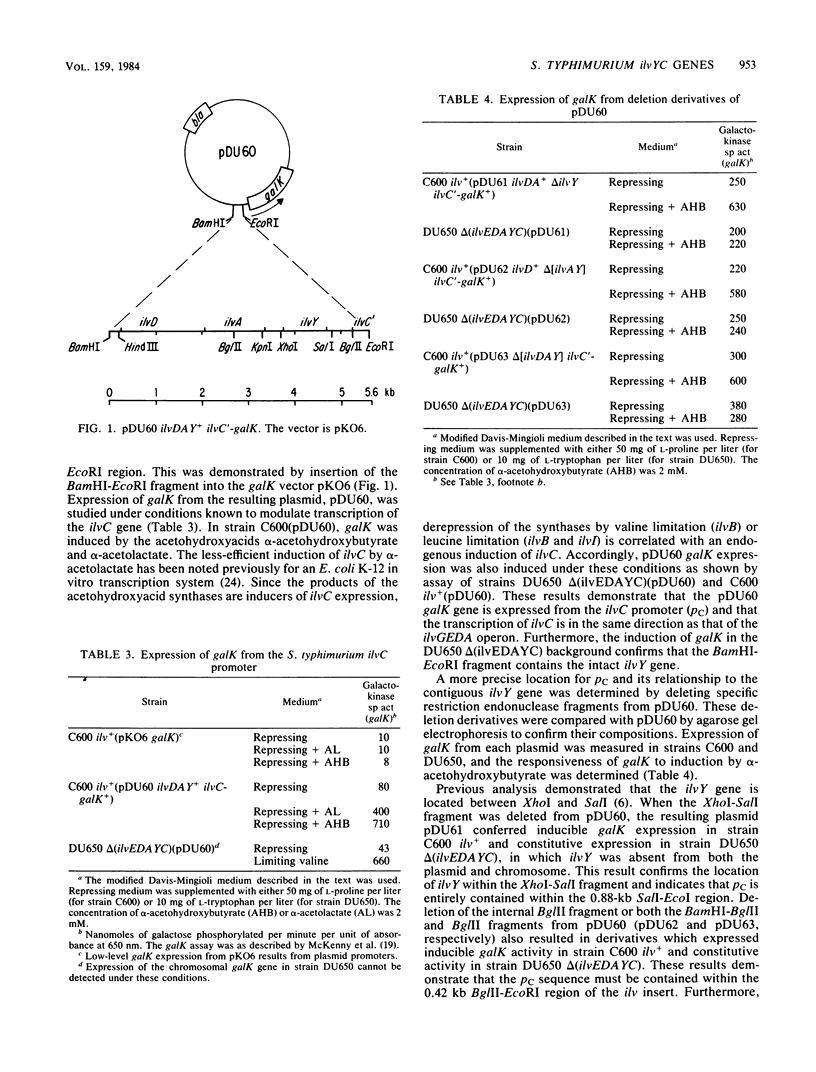
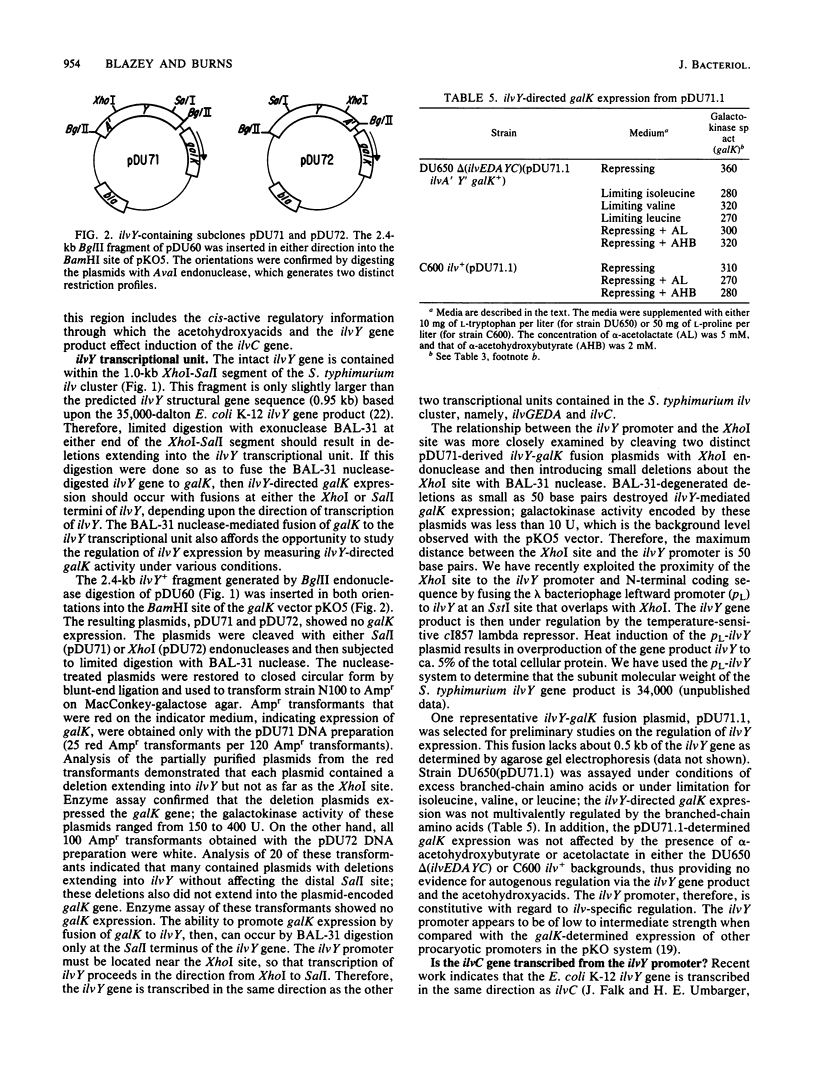
The Salmonella typhimurium LT2 ilvYC genes were studied by fusion of each gene to the Escherichia coli K-12 galK gene. The expression of ilvY and ilvC could then be determined by measurement of the galK-encoded galactokinase enzyme. The promoter for ilvC, pC, was located by this technique to a 0.42-kilobase BglII-EcoRI fragment of the S. typhimurium ilvGEDAYC gene cluster. This sequence was completely sufficient for alpha-acetohydroxyacid-inducible galK expression. The ilvY gene was located within a 1.0-kilobase XhoI-SalI fragment. ilvY gene expression was constitutive with respect to ilv-specific control signals. The ilvY gene was transcribed in the same direction as the other two transcriptional units in the ilvGEDAYC gene cluster, ilvGEDA and ilvC. Transcription of the ilvC gene was completely dependent upon the activity of its own promoter, pC, and independent from transcription of the ilvY gene. The role of the intervening region between ilvY and ilvC in regulation of ilvC expression was explored.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Ratzkin B., Umbarger H. E. The metabolism of valine and isoleucine in Escherichia coli. XVII. The role of induction in the derepression of acetohydroxy acid isomeroreductase. Biochem Biophys Res Commun. 1969 Dec 4;37(6):902–908. doi: 10.1016/0006-291x(69)90216-2. [DOI] [PubMed] [Google Scholar]
- Arfin S. M., Umbarger H. E. Purification and properties of the acetohydroxy acid isomeroreductase of Salmonella typhimurium. J Biol Chem. 1969 Mar 10;244(5):1118–1127. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazey D. L., Burns R. O. Gene ilvY of Salmonella typhimurium. J Bacteriol. 1980 Jun;142(3):1015–1018. doi: 10.1128/jb.142.3.1015-1018.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazey D. L., Burns R. O. Genetic organization of the Salmonella typhimurium ilv gene cluster. Mol Gen Genet. 1979;177(1):1–11. doi: 10.1007/BF00267247. [DOI] [PubMed] [Google Scholar]
- Blazey D. L., Kim R., Burns R. O. Molecular cloning and expression of the ilvGEDAY genes from Salmonella typhimurium. J Bacteriol. 1981 Aug;147(2):452–462. doi: 10.1128/jb.147.2.452-462.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray H. B., Jr, Ostrander D. A., Hodnett J. L., Legerski R. J., Robberson D. L. Extracellular nucleases of Pseudomonas BAL 31. I. Characterization of single strand-specific deoxyriboendonuclease and double-strand deoxyriboexonuclease activities. Nucleic Acids Res. 1975 Sep;2(9):1459–1492. doi: 10.1093/nar/2.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofler J. G., Decedue C. J., Luginbuhl G. H., Reynolds J. A., Burns R. O. The subunit structure of alpha-acetohydroxyacid isomeroreductase from Salmonella typhimurium. J Biol Chem. 1975 Feb 10;250(3):877–882. [PubMed] [Google Scholar]
- Housley P. R., Leavitt A. D., Whitfield H. J. Genetic analysis of a temperature-sensitive Salmonella typhimurium rho mutant with an altered rho-associated polycytidylate-dependent adenosine triphosphatase activity. J Bacteriol. 1981 Jul;147(1):13–24. doi: 10.1128/jb.147.1.13-24.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Reichardt K., Botstein D. Inversions and deletions of the Salmonella chromosome generated by the translocatable tetracycline resistance element Tn10. J Mol Biol. 1979 Jan 5;127(1):89–115. doi: 10.1016/0022-2836(79)90461-3. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Hodnett J. L., Gray H. B., Jr Extracellular nucleases of pseudomonas BAL 31. III. Use of the double-strand deoxyriboexonuclease activity as the basis of a convenient method for the mapping of fragments of DNA produced by cleavage with restriction enzymes. Nucleic Acids Res. 1978 May;5(5):1445–1464. doi: 10.1093/nar/5.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorkle G. M., Leathers T. D., Umbarger H. E. Physical organization of the ilvEDAC genes of Escherichia coli strain K-12. Proc Natl Acad Sci U S A. 1978 Jan;75(1):89–93. doi: 10.1073/pnas.75.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Taillon M. P., Gotto D. A., Lawther R. P. The DNA sequence of the promoter-attenuator of the ilvGEDA operon of Salmonella typhimurium. Nucleic Acids Res. 1981 Jul 24;9(14):3419–3432. doi: 10.1093/nar/9.14.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M. D., Wild J., Umbarger H. E. Positive control of ilvC expression in Escherichia coli K-12; identification and mapping of regulatory gene ilvY. J Bacteriol. 1979 Sep;139(3):1014–1020. doi: 10.1128/jb.139.3.1014-1020.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Wild J., Smith J. M., Umbarger H. E. In vitro synthesis of beta-galactosidase with ilv-lac fusion deoxyribonucleic acid as template. J Bacteriol. 1977 Dec;132(3):876–883. doi: 10.1128/jb.132.3.876-883.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Hogness D. S. The enzymes of the galactose operon in Escherichia coli. IV. The frequencies of translation of the terminal cistrons in the operon. J Biol Chem. 1969 Apr 25;244(8):2143–2148. [PubMed] [Google Scholar]


