Abstract
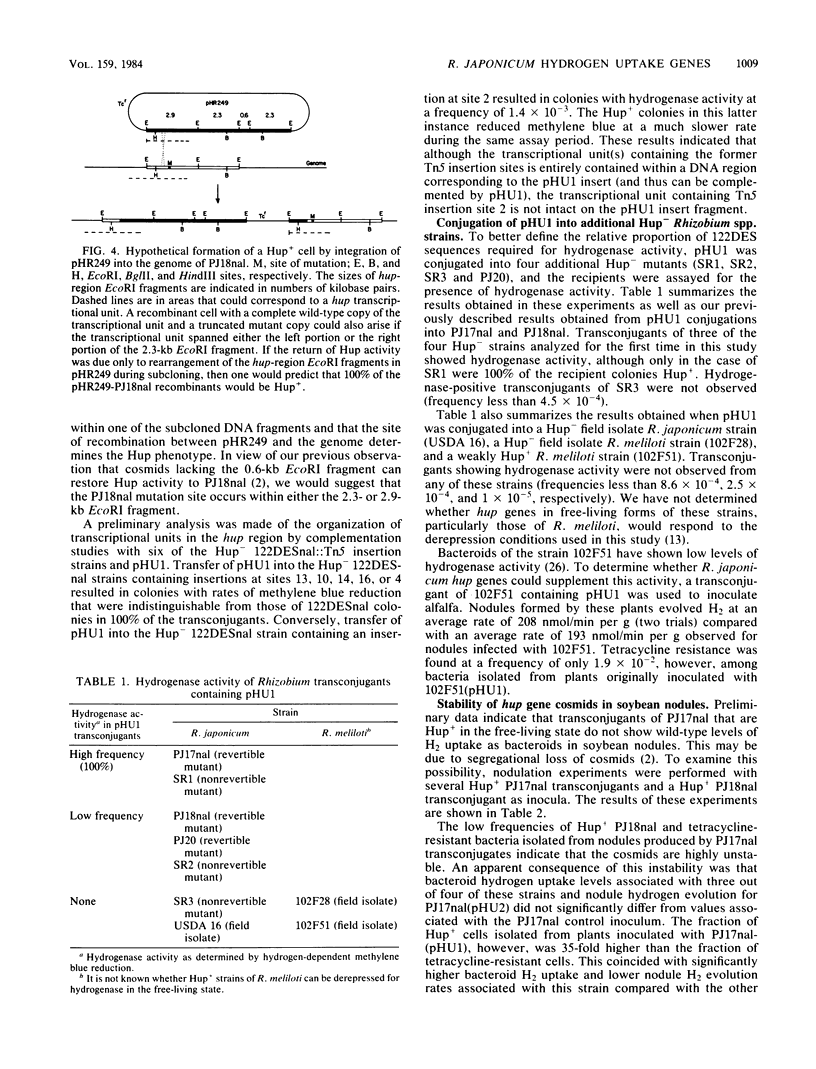
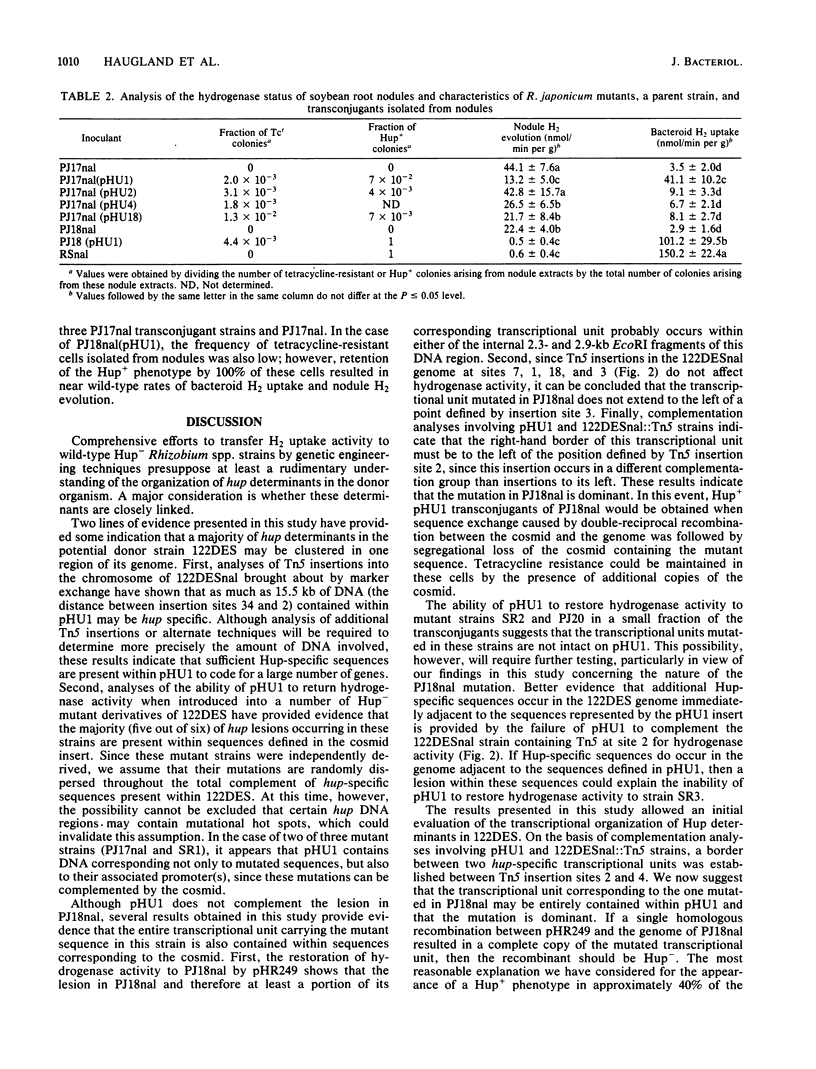
Recombinant cosmids from a gene library of the DNA from Hup+ Rhizobium japonicum 122DES previously have been shown to restore hydrogenase activity when transferred by conjugation into certain Hup- mutants of R. japonicum. We generated a restriction map covering 32.2 kilobases of this cosmid DNA. At least 25.3 kilobases of the cosmid pHU1 were shown to have the same arrangement as those in the genome of strain 122DES. Analysis of Tn5 insertions into the 122DES genome indicates that hup-specific sequences occur in a region spanning about 15 kilobases of insert DNA within pHU1. Introduction of pHU1 into five out of six R. japonicum Hup- mutants resulted in a Hup+ phenotype in some transconjugants. Three of the mutations appear to be in transcriptional units completely contained within pHU1, whereas the other two must be in genes that are at least partially contained within pHU1. pBR235 derivatives containing fragments of hup DNA can be transferred into the R. japonicum Hup- mutant PJ18nal if the derivatives contain a region of homology with the R. japonicum genome. The hup mutation in strain PJ18nal appears to be dominant. The hup genes in R. japonicum strain 122DES appear to be organized in at least two, and probably three, transcriptional units.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantrell M. A., Haugland R. A., Evans H. J. Construction of a Rhizobium japonicum gene bank and use in isolation of a hydrogen uptake gene. Proc Natl Acad Sci U S A. 1983 Jan;80(1):181–185. doi: 10.1073/pnas.80.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Schilling-Cordaro C., Mergia A., Houck C. M. A new technique for genetic engineering of Agrobacterium Ti plasmid. Plasmid. 1983 Jul;10(1):21–30. doi: 10.1016/0147-619x(83)90054-9. [DOI] [PubMed] [Google Scholar]
- Corbin D., Barran L., Ditta G. Organization and expression of Rhizobium meliloti nitrogen fixation genes. Proc Natl Acad Sci U S A. 1983 May;80(10):3005–3009. doi: 10.1073/pnas.80.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin D., Ditta G., Helinski D. R. Clustering of nitrogen fixation (nif) genes in Rhizobium meliloti. J Bacteriol. 1982 Jan;149(1):221–228. doi: 10.1128/jb.149.1.221-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Drevon J. J., Frazier L., Russell S. A., Evans H. J. Respiratory and Nitrogenase Activities of Soybean Nodules Formed by Hydrogen Uptake Negative (Hup) Mutant and Revertant Strains of Rhizobium japonicum Characterized by Protein Patterns. Plant Physiol. 1982 Nov;70(5):1341–1346. doi: 10.1104/pp.70.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R. A., Hanus F. J., Cantrell M. A., Evans H. J. Rapid Colony Screening Method for Identifying Hydrogenase Activity in Rhizobium japonicum. Appl Environ Microbiol. 1983 Mar;45(3):892–897. doi: 10.1128/aem.45.3.892-897.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R., Verma D. P. Interspecific plasmid and genomic DNA sequence homologies and localization of nif genes in effective and ineffective strains of Rhizobium japonicum. J Mol Appl Genet. 1981;1(3):205–217. [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Lepo J. E., Hickok R. E., Cantrell M. A., Russell S. A., Evans H. J. Revertible hydrogen uptake-deficient mutants of Rhizobium japonicum. J Bacteriol. 1981 May;146(2):614–620. doi: 10.1128/jb.146.2.614-620.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbird R. M., Atkins C. A., Pate J. S., Sanford P. Significance of hydrogen evolution in the carbon and nitrogen economy of nodulated cowpea. Plant Physiol. 1983 Jan;71(1):122–127. doi: 10.1104/pp.71.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruiz-Argüeso T., Maier R. J., Evans H. J. Hydrogen Evolution from Alfalfa and Clover Nodules and Hydrogen Uptake by Free-Living Rhizobium meliloti. Appl Environ Microbiol. 1979 Mar;37(3):582–587. doi: 10.1128/aem.37.3.582-587.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]