Abstract
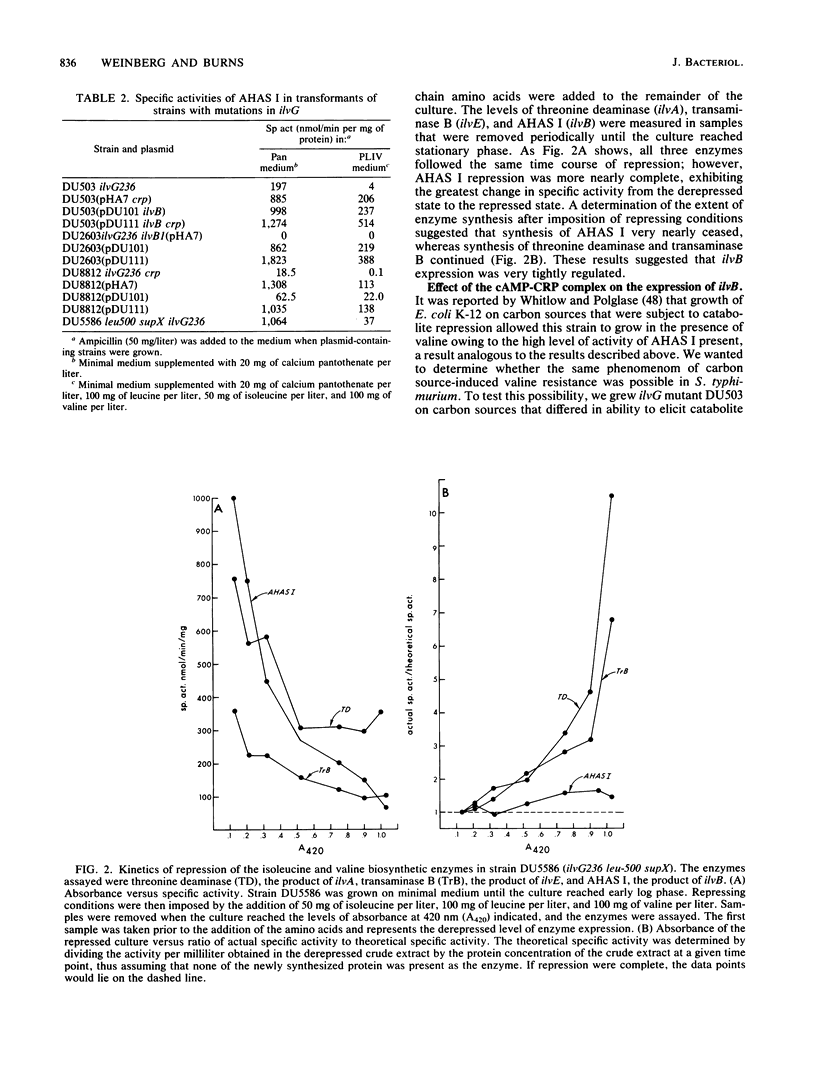
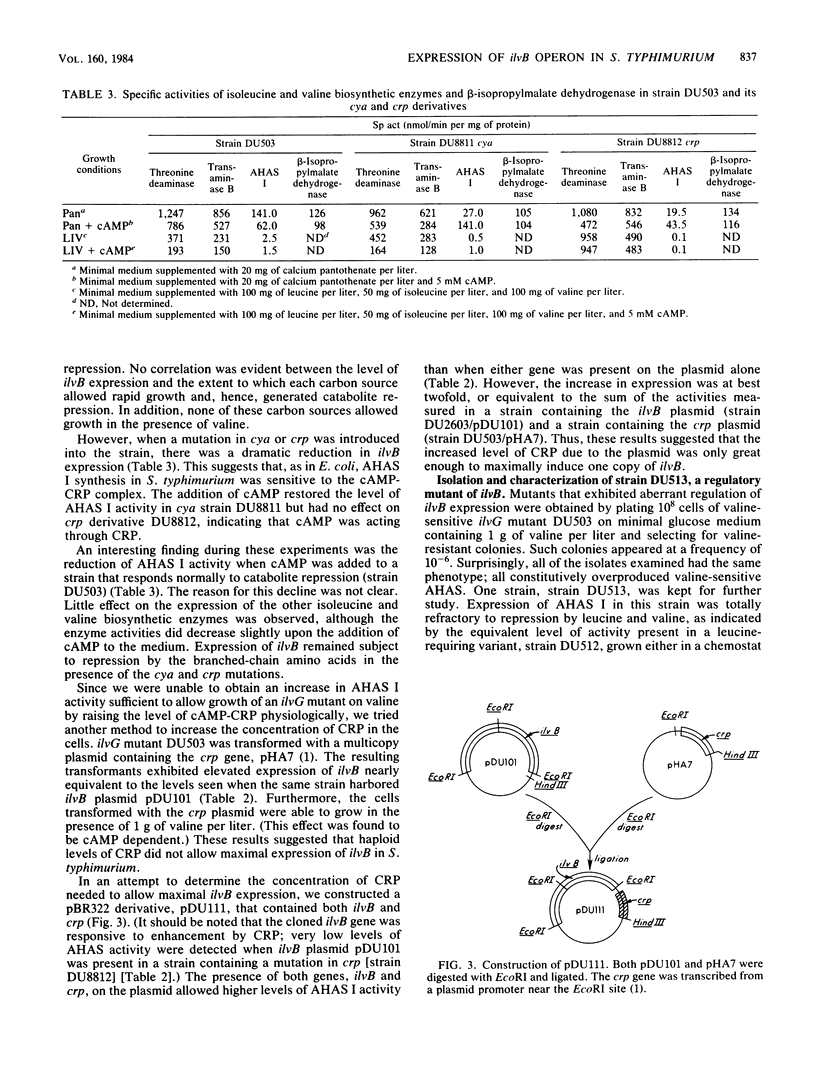
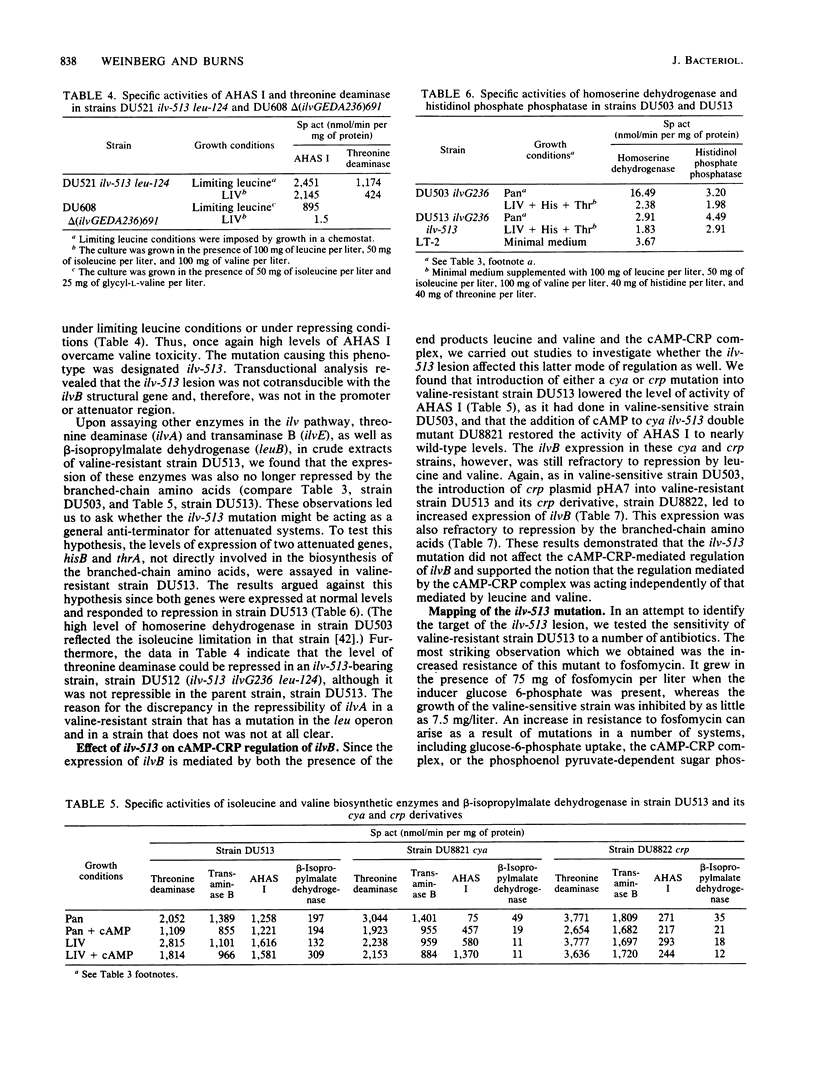
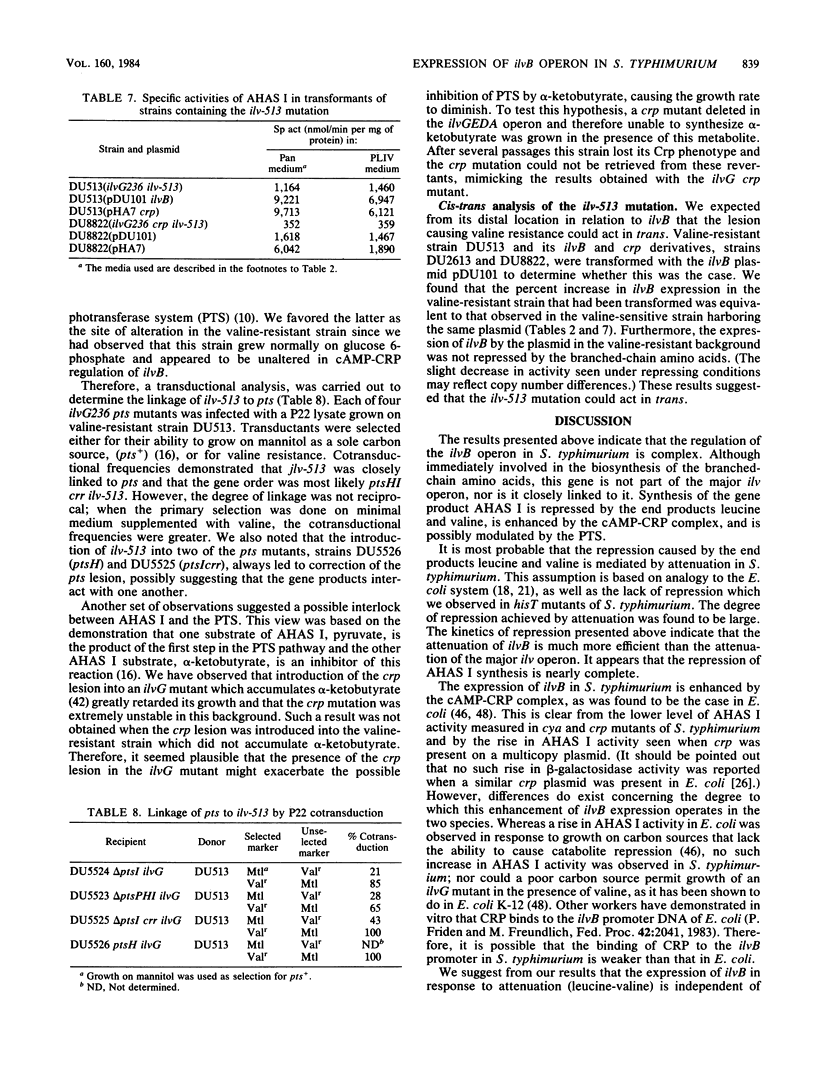
The ilvB gene of Salmonella typhimurium encodes the valine-sensitive form of acetohydroxy acid synthase, acetohydroxy acid synthase I, which catalyzes the first step in the parallel biosynthesis of isoleucine and valine. Although nearly all of the other genes involved in this pathway are clustered at minute 83, ilvB was found to lie at minute 80.5. Expression of ilvB was shown to be nearly completely repressed by the end products leucine and valine. Studies in which we used strains with mutations in cya (adenylate cyclase) and crp (cAMP receptor protein) demonstrated that synthesis of acetohydroxy acid synthase I is enhanced by the cAMP-cAMP receptor protein complex. Although no stimulation was achieved by growth on poor carbon sources, introduction of crp on a multicopy plasmid led to markedly increased expression. Strains of S. typhimurium lacking valine-resistant acetohydroxy acid synthase II (ilvG) are like Escherichia coli K-12 in that they are not able to grow in the presence of L-valine owing to a conditional isoleucine auxotrophy. The valine toxicity of these ilvG mutants of S. typhimurium was overcome by increasing the level of acetohydroxy acid synthase I. Enzyme activity could be elevated either by maximally derepressing expression with severe leucine limitation, by introduction of either ilvB or crp on a multicopy plasmid, or by the presence of the ilv-513 mutation. This mutation, which is closely linked to genes encoding the phosphoenol pyruvate:sugar phosphotransferase system (pts), causes highly elevated expression of ilvB that is refractory to repression by leucine and valine, as is the major ilv operon. The response of ilvB to the cAMP-cAMP receptor protein complex was not affected by this lesion. Data obtained by using this mutant led us to propose that the two modes of regulation act independently. We also present some evidence which suggests that ilvB expression may be affected by the phosphoenol pyruvate:sugar phosphotransferase system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Fujimoto S., Ozaki N. Molecular cloning and nucleotide sequencing of the gene for E. coli cAMP receptor protein. Nucleic Acids Res. 1982 Feb 25;10(4):1345–1361. doi: 10.1093/nar/10.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUERLE R. H., FRUENDLICH M., STORMER F. C., UMBARGER H. E. CONTROL OF ISOLEUCINE, VALINE AND LEUCINE BIOSYNTHESIS. II. ENDPRODUCT INHIBITION BY VALINE OF ACETOHYDROXY ACID SYNTHETASE IN SALMONELLA TYPHIMURIUM. Biochim Biophys Acta. 1964 Oct 23;92:142–149. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazey D. L., Burns R. O. Genetic organization of the Salmonella typhimurium ilv gene cluster. Mol Gen Genet. 1979;177(1):1–11. doi: 10.1007/BF00267247. [DOI] [PubMed] [Google Scholar]
- Botsford J. L. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981 Dec;45(4):620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunovskis I., Burns R. O. Growth of coliphage T7 in Salmonella typhimurium. J Virol. 1973 May;11(5):621–629. doi: 10.1128/jvi.11.5.621-629.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen R. K., Duester G. L., Holmes W. M., Young J. M. Organization of transfer ribonucleic acid genes in the Escherichia coli chromosome. J Bacteriol. 1980 Dec;144(3):1083–1093. doi: 10.1128/jb.144.3.1083-1093.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaro J. C., Roseman S. Deletion mapping of the genes coding for HPr and enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):17–29. doi: 10.1128/jb.112.1.17-29.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J., Dondon L., Danchin A. 2-Ketobutyrate: a putative alarmone of Escherichia coli. Mol Gen Genet. 1983;190(3):452–458. doi: 10.1007/BF00331076. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan D. E., Wechsler J. A. An assay for transaminase B enzyme activity in Escherichia coli K-12. Anal Biochem. 1973 Jan;51(1):67–79. doi: 10.1016/0003-2697(73)90453-3. [DOI] [PubMed] [Google Scholar]
- Friden P., Newman T., Freundlich M. Nucleotide sequence of the ilvB promoter-regulatory region: a biosynthetic operon controlled by attenuation and cyclic AMP. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6156–6160. doi: 10.1073/pnas.79.20.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glesyna M. L., Bolshakova T. N., Gershanovitch V. N. Effect of ptsI and ptsH mutations on initiation of transcription of the Escherichia coli lactose operon. Mol Gen Genet. 1983;190(3):417–420. doi: 10.1007/BF00331070. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C. A., Hatfield G. W. Nucleotide sequence of the ilvB multivalent attenuator region of Escherichia coli K12. Nucleic Acids Res. 1983 Jan 11;11(1):127–139. doi: 10.1093/nar/11.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S. Novel F prime factors able to replicate in Escherichia coli Hfr strains. Proc Natl Acad Sci U S A. 1976 Jan;73(1):198–202. doi: 10.1073/pnas.73.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph E., Bernsley C., Guiso N., Ullmann A. Multiple regulation of the activity of adenylate cyclase in Escherichia coli. Mol Gen Genet. 1982;185(2):262–268. doi: 10.1007/BF00330796. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Reichardt K., Botstein D. Inversions and deletions of the Salmonella chromosome generated by the translocatable tetracycline resistance element Tn10. J Mol Biol. 1979 Jan 5;127(1):89–115. doi: 10.1016/0022-2836(79)90461-3. [DOI] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawther R. P., Hatfield G. W. Multivalent translational control of transcription termination at attenuator of ilvGEDA operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1862–1866. doi: 10.1073/pnas.77.4.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. doi: 10.1093/genetics/48.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mukai F. H., Margolin P. ANALYSIS OF UNLINKED SUPPRESSORS OF AN O degrees MUTATION IN SALMONELLA. Proc Natl Acad Sci U S A. 1963 Jul;50(1):140–148. doi: 10.1073/pnas.50.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargang F. E., Subrahmanyam C. S., Umbarger H. E. Nucleotide sequence of ilvGEDA operon attenuator region of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1823–1827. doi: 10.1073/pnas.77.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman T. C., Levinthal M. A new map location for the ilvB locus of Escherichia coli. Genetics. 1980 Sep;96(1):59–77. doi: 10.1093/genetics/96.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J. P., Freundlich M. Temperature-sensitive growth inhibition by valine in Salmonella typhimurium: alteration of one form of acetohydroxy acid synthetase. J Bacteriol. 1973 Oct;116(1):98–106. doi: 10.1128/jb.116.1.98-106.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primerano D. A., Burns R. O. Metabolic basis for the isoleucine, pantothenate or methionine requirement of ilvG strains of Salmonella typhimurium. J Bacteriol. 1982 Jun;150(3):1202–1211. doi: 10.1128/jb.150.3.1202-1211.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Freundlich M. Regulation of cyclic AMP of the ilvB-encoded biosynthetic acetohydroxy acid synthase in Escherichia coli K-12. Mol Gen Genet. 1980 Apr;178(1):179–183. doi: 10.1007/BF00267227. [DOI] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Whitlow K. J., Polglase W. J. Relaxation of catabolite repression and loss of valine sensitivity in Escherichia coli K-12. FEBS Lett. 1974 Jul 1;43(1):64–66. doi: 10.1016/0014-5793(74)81106-3. [DOI] [PubMed] [Google Scholar]
- van Zoelen E. J., van Dijck P. W., de Kruijff B., Verkleij A. J., van Deenen L. L. Effect of glycophorin incorporation on the physico-chemical properties of phospholipid bilayers. Biochim Biophys Acta. 1978 Dec 4;514(1):9–24. doi: 10.1016/0005-2736(78)90073-1. [DOI] [PubMed] [Google Scholar]


