Abstract
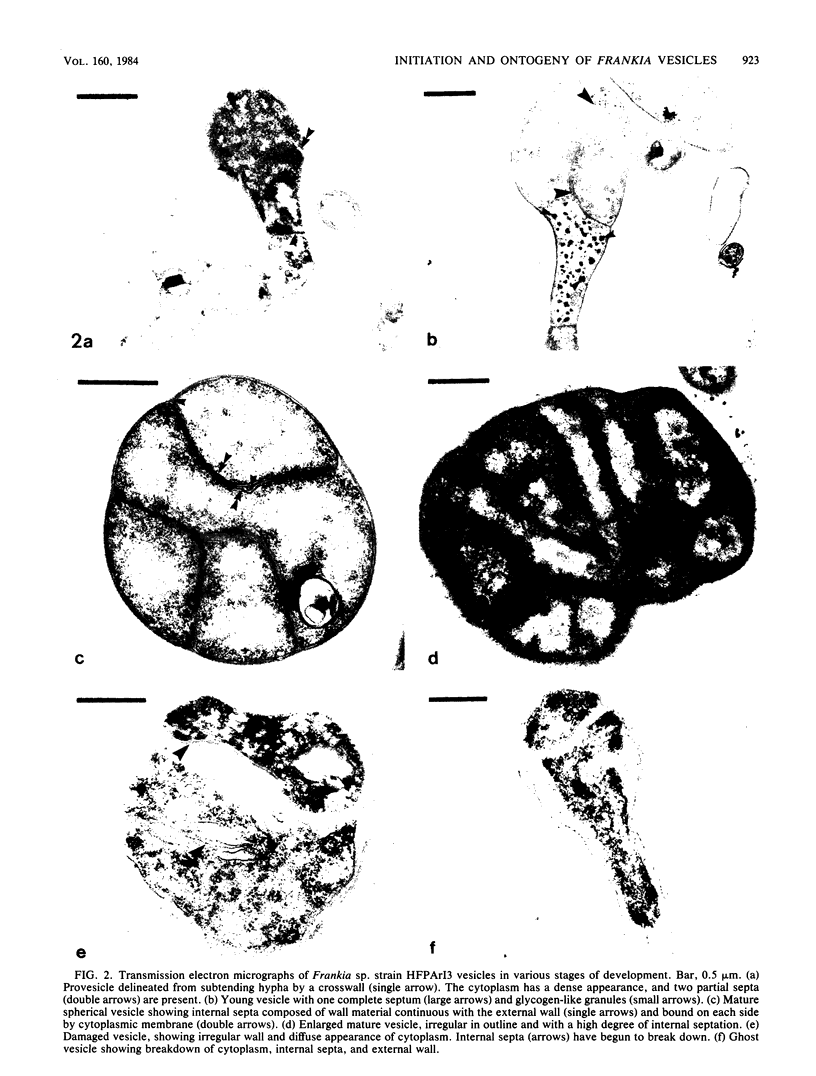
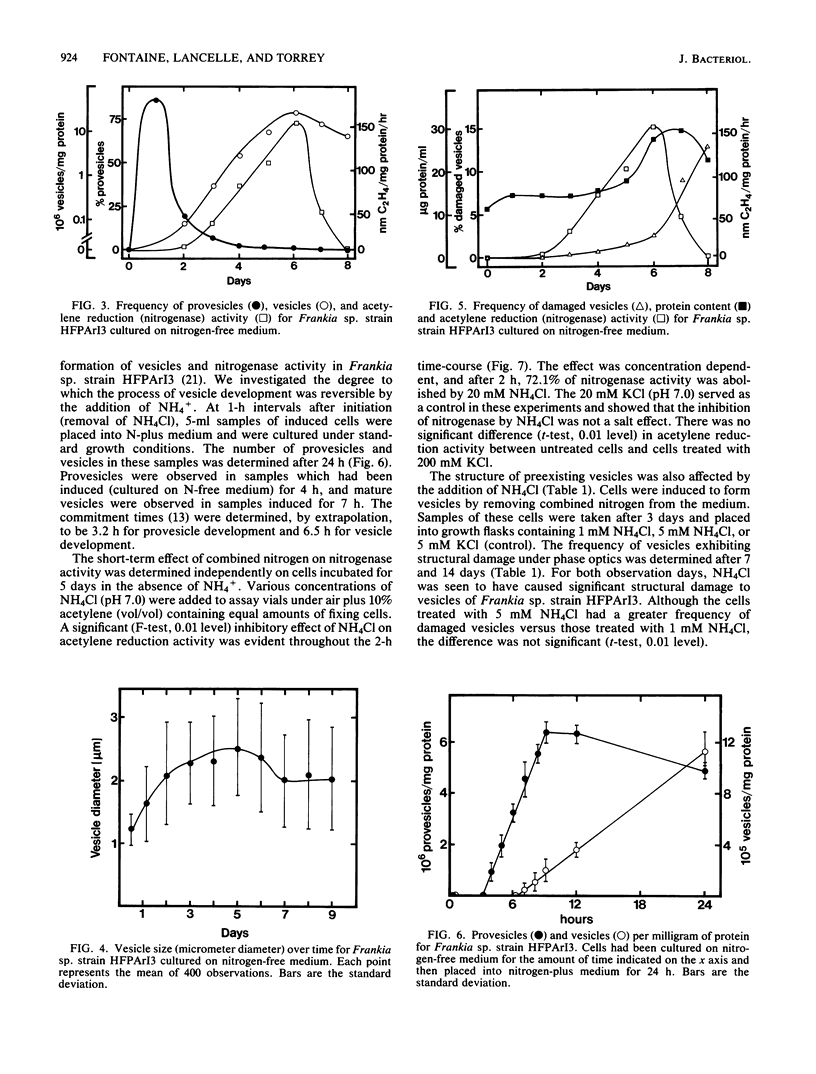
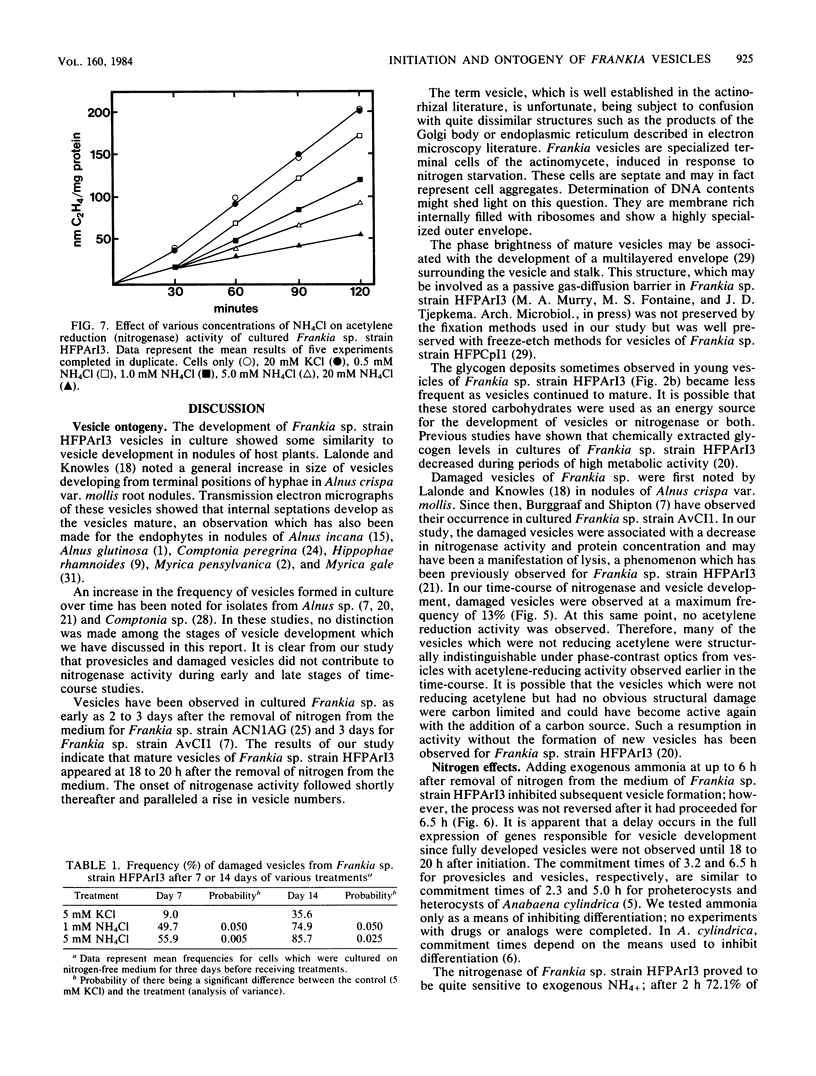
Removal of combined nitrogen from the medium of Frankia sp. strain HFPArI3 induced the formation of specialized structures, called vesicles, which are the proposed site of nitrogen fixation. After 5 to 6 h of culture on N-free medium, newly formed vesicles, termed provesicles, arose from the tips of some hyphae. These structures were spherical, phase dark, ca. 1.5 to 2.0 micron in diameter, and were not associated with acetylene reduction (nitrogenase) activity. Provesicles reached their greatest frequency after ca. 24 h of N-free culture. Provesicles increased in size to become mature vesicles which first appeared after 18 to 20 h of N-free culture. They were ca. 2.5 micron in diameter, phase bright, and reached their greatest frequency after 5 to 6 days, at which time nitrogenase activity peaked. Some vesicles eventually became damaged structurally and took on the appearance of ghosts. Transmission electron micrographs revealed an increase in size from provesicle to mature vesicle. Also evident with the micrographs were the presence of a septum between the young provesicle and parental hypha, the presence of glycogen in some young vesicles, the development of internal septations as vesicles matured, and the degradation of cytoplasm and internal septae in ghost vesicles. The extent to which the formation of vesicles is reversible by the addition of NH4+ was investigated. Commitment times of 3.2 and 6.5 h were obtained for provesicles and vesicles, respectively. A concentration-dependent inhibition of nitrogenase by NH4+ was demonstrated. The structure of preexisting vesicles was also affected by addition of NH4+ to the culture medium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKING J. H., DE BOER W. E., HOUWINK A. L. ELECTRON MICROSCOPY OF THE ENDOPHYTE OF ALNUS GLUTINOSA. Antonie Van Leeuwenhoek. 1964;30:343–376. doi: 10.1007/BF02046749. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bradley S., Carr N. G. Heterocyst and nitrogenase development in Anabaena cylindrica. J Gen Microbiol. 1976 Sep;96(1):175–184. doi: 10.1099/00221287-96-1-175. [DOI] [PubMed] [Google Scholar]
- Gauthier D., Diem H. G., Dommergues Y. In vitro nitrogen fixation by two actinomycete strains isolated from casuarina nodules. Appl Environ Microbiol. 1981 Jan;41(1):306–308. doi: 10.1128/aem.41.1.306-308.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. C., Meyer C. M., Vignais P. M. Nitrogenase from the photosynthetic bacterium Rhodopseudomonas capsulata: purification and molecular properties. J Bacteriol. 1982 Feb;149(2):708–717. doi: 10.1128/jb.149.2.708-717.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard K. S., Hales B. J., Socolofsky M. D. Nitrogen fixation and ammonia switch-off in the photosynthetic bacterium Rhodopseudomonas viridis. J Bacteriol. 1983 Jul;155(1):107–112. doi: 10.1128/jb.155.1.107-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto R. H., Ludden P. W. Effect of ammonia, darkness, and phenazine methosulfate on whole-cell nitrogenase activity and Fe protein modification in Rhodospirillum rubrum. J Bacteriol. 1984 May;158(2):713–720. doi: 10.1128/jb.158.2.713-720.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laane C., Krone W., Konings W., Haaker H., Veeger C. Short-term effect of ammonium chloride on nitrogen fixation by Azotobacter vinelandii and by bacteroids of Rhizobium leguminosarum. Eur J Biochem. 1980 Jan;103(1):39–46. doi: 10.1111/j.1432-1033.1980.tb04286.x. [DOI] [PubMed] [Google Scholar]
- Lalonde M., Knowles R. Ultrastructure of the Alnus crispa var. mollis Fern. root nodule endophyte. Can J Microbiol. 1975 Jul;21(7):1058–1080. doi: 10.1139/m75-157. [DOI] [PubMed] [Google Scholar]
- Neilson A. H., Nordlund S. Regulation of nitrogenase synthesis in intact cells of Rhodospirillum rubrum: inactivation of nitrogen fixation by ammonia, L-glutamine and L-asparagine. J Gen Microbiol. 1975 Nov;91(1):53–62. doi: 10.1099/00221287-91-1-53. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Tjepkema J. D., Ormerod W., Torrey J. G. Factors affecting vesicle formation and acetylene reduction (nitrogenase activity) in Frankia sp. CpI1. Can J Microbiol. 1981 Aug;27(8):815–823. doi: 10.1139/m81-126. [DOI] [PubMed] [Google Scholar]
- Torrey J. G., Tjepkema J. D., Turner G. L., Bergersen F. J., Gibson A. H. Dinitrogen Fixation by Cultures of Frankia sp CpIl Demonstrated by N(2) Incorporation. Plant Physiol. 1981 Oct;68(4):983–984. doi: 10.1104/pp.68.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G., Castillo F. Regulatory properties of the nitrogenase from Rhodopseudomonas palustris. Arch Microbiol. 1978 Apr 27;117(1):53–60. doi: 10.1007/BF00689351. [DOI] [PubMed] [Google Scholar]