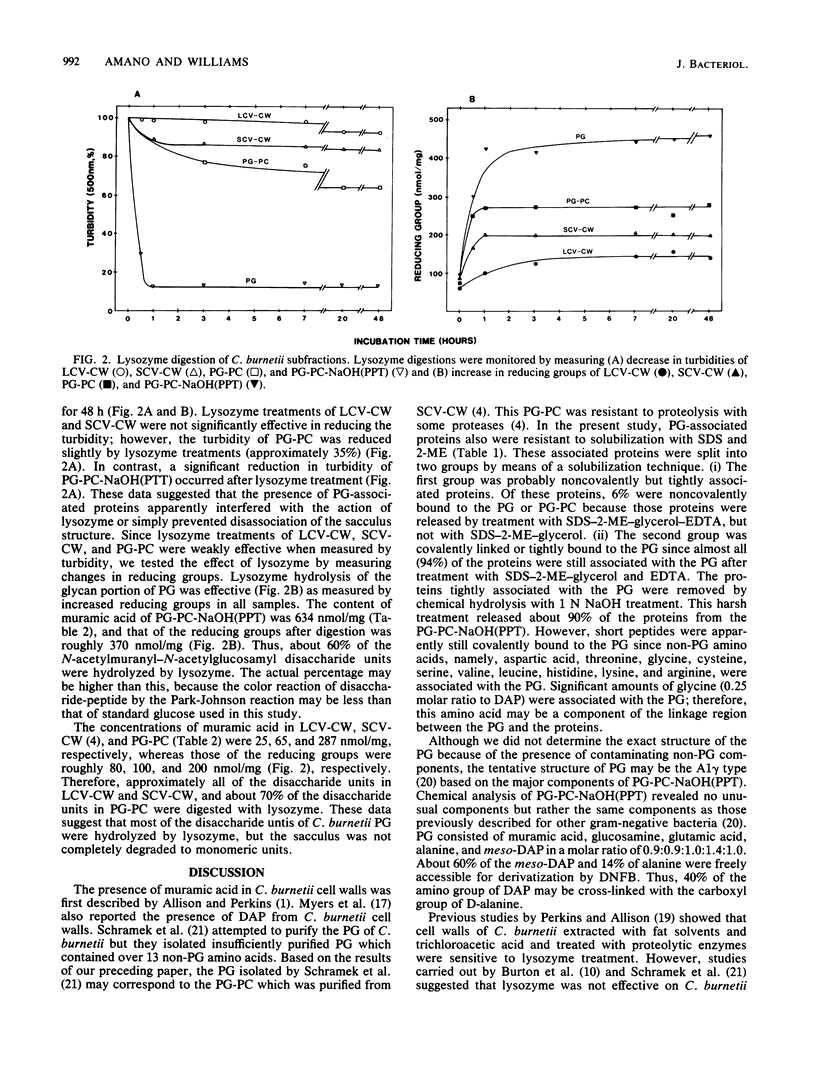
Abstract
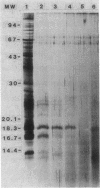
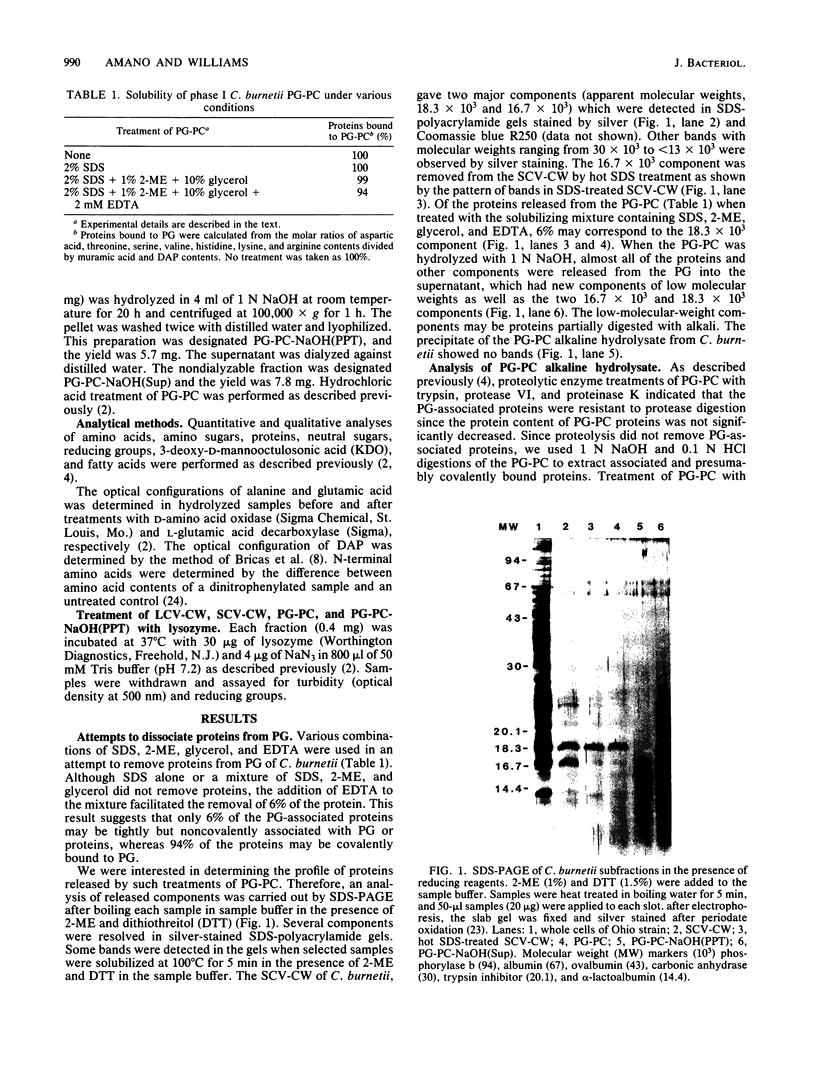
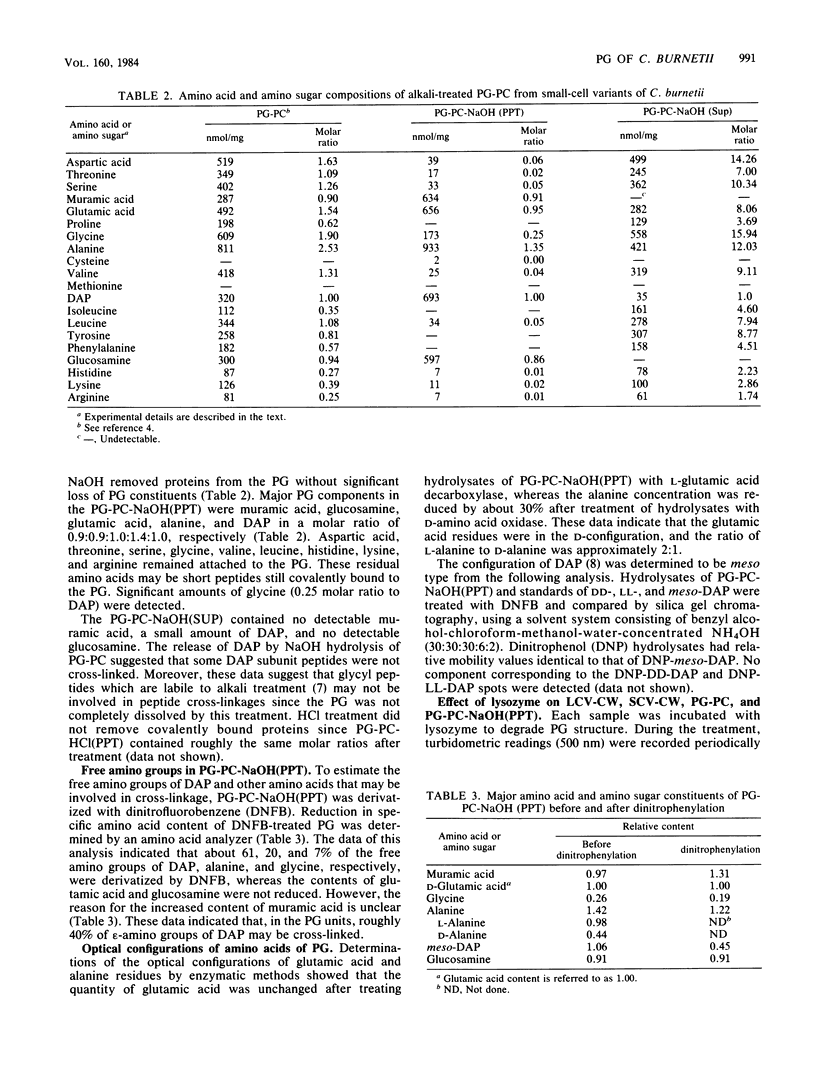
The protease-resistant proteins associated with the peptidoglycan (PG) of the phase I small-cell variant Coxiella burnetii were either partially released from the PG by boiling the PG-protein complex (PG-PC) in sodium dodecyl sulfate containing 2-mercaptoethanol and EDTA or totally released by 1 N NaOH hydrolysis at 23 degrees C. An 18,300-dalton protein was released from the PG-PC under reducing conditions, whereas 1 N NaOH treatment extracted PG-associated proteins without apparent dissolution of the PG. Purified PG was composed of muramic acid, glucosamine, glutamic acid, alanine, and meso-diaminopimelic acid in a molar ratio of 0.9:0.9:1.0:1.4:1.0. Lysozyme hydrolysis of cell walls, PG-PC, and purified PG caused an increase in reducing groups which correlated with roughly 60 to 100% digestion of disaccharides. There was no significant decrease in turbidity during lysozyme hydrolysis of cell walls and PG-PC; however, hydrolysis of purified PG caused about 90% decrease in turbidity. Approximately 60% of the meso-diaminopimelic acid groups of PG were not susceptible to dinitrophenylation, thus, demonstrating an apparent contribution of PG-associated proteins, rather than cross-linkage between peptides, to sacculus rigidity of cell wall and PG-PC. This association of PG and protease-resistant covalently bound proteins may be important structural and functional determiners of resistance to both environmental conditions and intracellular digestion of C. burnetii by eucaryotic cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANACKER R. L., FUKUSHI K., PICKENS E. G., LACKMAN D. B. ELECTRON MICROSCOPIC OBSERVATIONS OF THE DEVELOPMENT OF COXIELLA BURNETII IN THE CHICK YOLK SAC. J Bacteriol. 1964 Oct;88:1130–1138. doi: 10.1128/jb.88.4.1130-1138.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K., Williams J. C., McCaul T. F., Peacock M. G. Biochemical and immunological properties of Coxiella burnetii cell wall and peptidoglycan-protein complex fractions. J Bacteriol. 1984 Dec;160(3):982–988. doi: 10.1128/jb.160.3.982-988.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K., Williams J. C. Partial characterization of peptidoglycan-associated proteins of Legionella pneumophila. J Biochem. 1983 Aug;94(2):601–606. doi: 10.1093/oxfordjournals.jbchem.a134392. [DOI] [PubMed] [Google Scholar]
- Amano K., Williams J. C. Peptidoglycan of Legionella pneumophila: apparent resistance to lysozyme hydrolysis correlates with a high degree of peptide cross-linking. J Bacteriol. 1983 Jan;153(1):520–526. doi: 10.1128/jb.153.1.520-526.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y., Nakatani T., Nakayama K., Ito E. Occurrence of N-nonsubstituted glucosamine residues in peptidoglycan of lysozyme-resistant cell walls from Bacillus cereus. J Biol Chem. 1972 Oct 10;247(19):6312–6322. [PubMed] [Google Scholar]
- Archibald A. R., Baddiley J., Goundry J. The action of dilute alkali on the glycyl residues of staphylococcal peptidoglycan. Biochem J. 1970 Jan;116(2):313–315. doi: 10.1042/bj1160313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUMFITT W., WARDLAW A. C., PARK J. T. Development of lysozyme-resistance in Micrococcus lysodiekticus and its association with an increased O-acetyl content of the cell wall. Nature. 1958 Jun 28;181(4626):1783–1784. doi: 10.1038/1811783a0. [DOI] [PubMed] [Google Scholar]
- Bricas E., Ghuysen J. M., Dezélée P. The cell wall peptidoglycan of Bacillus megaterium KM. I. Studies on the stereochemistry of alpha, alpha'-diaminopimelic acid. Biochemistry. 1967 Aug;6(8):2598–2607. doi: 10.1021/bi00860a043. [DOI] [PubMed] [Google Scholar]
- Burton P. R., Stueckemann J., Paretsky D. Electron microscopy studies of the limiting layers of the rickettsia Coxiella burneti. J Bacteriol. 1975 Apr;122(1):316–324. doi: 10.1128/jb.122.1.316-324.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. W., Wilkinson S. G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol. 1965 Jun;39(3):385–399. doi: 10.1099/00221287-39-3-385. [DOI] [PubMed] [Google Scholar]
- KRAUSE R. M., MCCARTY M. Studies on the chemical structure of the streptococcal cell wall. I. The identification of a mucopeptide in the cell walls of groups A and A-variant streptococci. J Exp Med. 1961 Jul 1;114:127–140. doi: 10.1084/jem.114.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAVCHENKO A. T., GUDINA O. S., MULIUTIN V. N. [A study of the effect of antibiotics and specific sera on the development of viruses and rickettsia in tissue culture with the use of microcinematography. I. The effect of penicillin on psittacosis virus and Rickettsia burneti in tissue culture]. Vopr Virusol. 1961 May-Jun;7:300–306. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCaul T. F., Williams J. C. Developmental cycle of Coxiella burnetii: structure and morphogenesis of vegetative and sporogenic differentiations. J Bacteriol. 1981 Sep;147(3):1063–1076. doi: 10.1128/jb.147.3.1063-1076.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. R., ALLISON A. C. Cell-wall constituents of rickettsiae and psittacosis-lymphogranuloma organisms. J Gen Microbiol. 1963 Mar;30:469–480. doi: 10.1099/00221287-30-3-469. [DOI] [PubMed] [Google Scholar]
- PERKINS H. R. THE ACTION OF HOT FORMAMIDE ON BACTERIAL CELL WALLS. Biochem J. 1965 Jun;95:876–882. doi: 10.1042/bj0950876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Umemoto T., Ota T., Sagawa H., Kato K., Takada H., Tsujimoto M., Kawasaki A., Ogawa T., Harada K., Kotani S. Chemical and biological properties of a peptidoglycan isolated from Treponema pallidum kazan. Infect Immun. 1981 Feb;31(2):767–774. doi: 10.1128/iai.31.2.767-774.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]