Abstract
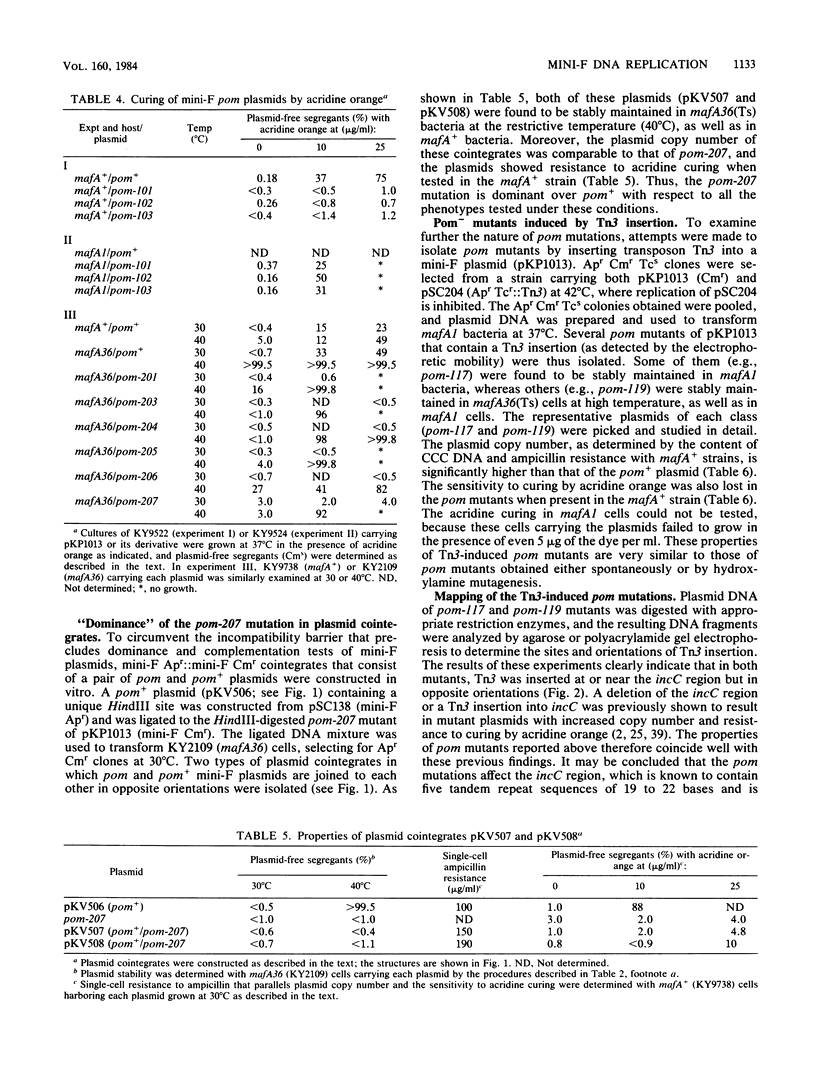
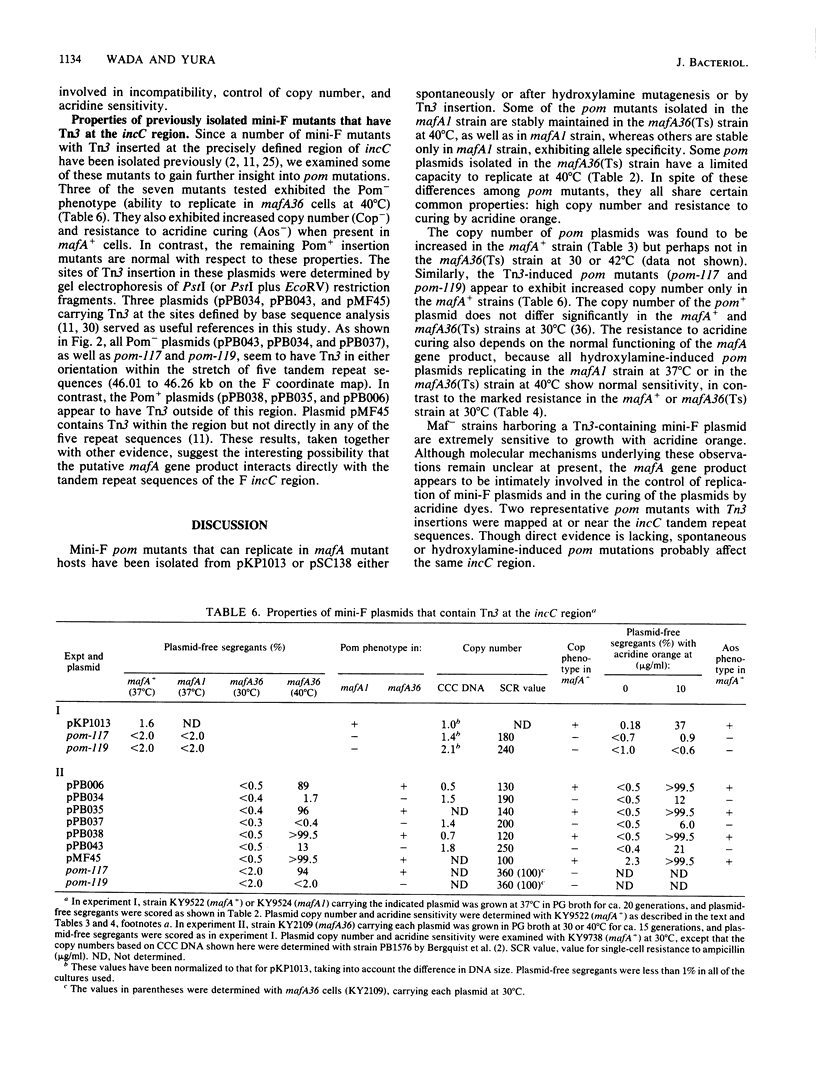
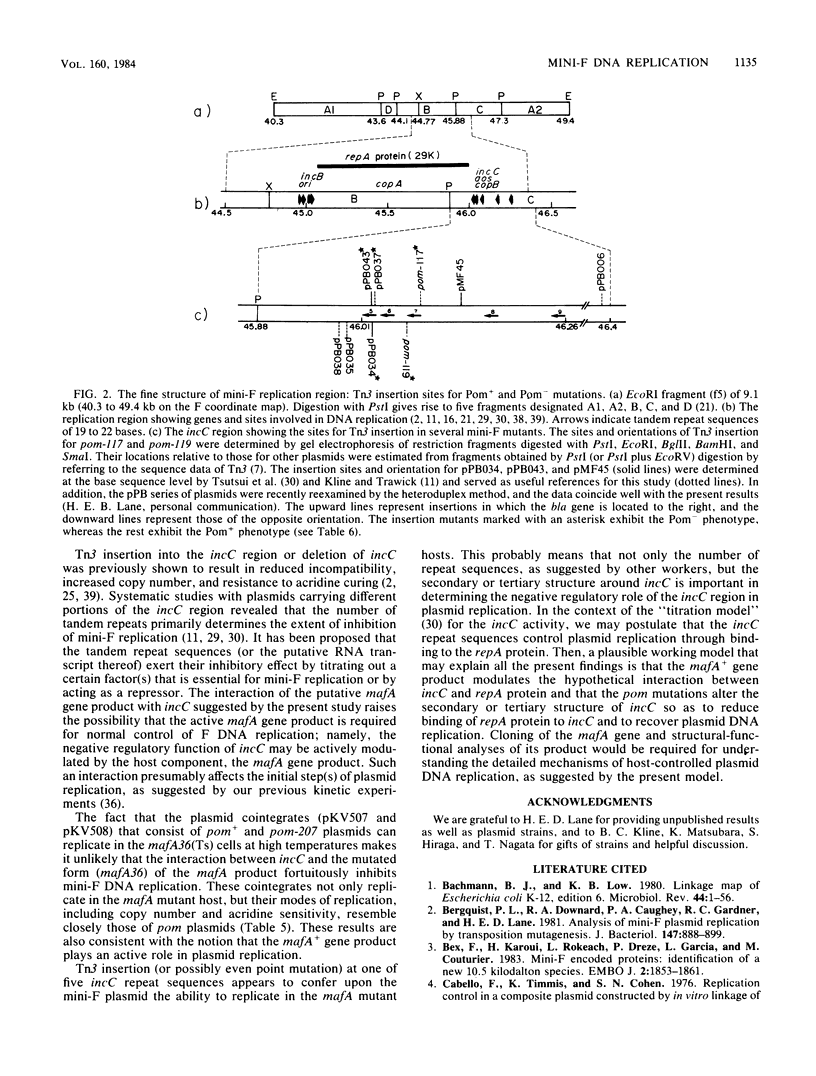
Replication of F (including mini-F) and some related plasmids is known to be specifically inhibited in mafA mutants of Escherichia coli K-12. We have now isolated and characterized mini-F mutants that can overcome the replication inhibition. Such plasmids, designated pom (permissive on maf), were obtained spontaneously or after mutagenesis with hydroxylamine or by transposon (Tn3) insertion. In addition to their ability to replicate in mafA mutant bacteria, the pom mutant plasmids exhibit an increased copy number and resistance to "curing" by acridine dye in the mafA+ host. In agreement with these results, Tn3-induced pom mutants were found to carry Tn3 inserted at the incC region of mini-F DNA, known to be involved in incompatibility, control of copy number, and sensitivity to acridine dye. Furthermore, three of the seven mini-F plasmids tested that carry Tn3 within the tandem repeat sequences of the incC region (previously isolated by other workers) exhibit all the phenotypes of pom plasmids, the ability to replicate in the mafA strain, and high copy number and acridine resistance in the mafA+ strain. The rest of the plasmids that contain Tn3 just outside the tandem repeats remain wild type in all these properties. These results strongly suggest that the putative mafA gene product of host bacteria controls mini-F replication through interaction with the incC region.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist P. L., Downard R. A., Caughey P. A., Gardner R. C., Lane H. E. Analysis of mini-F plasmid replication by transposition mutagenesis. J Bacteriol. 1981 Sep;147(3):888–899. doi: 10.1128/jb.147.3.888-899.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bex F., Karoui H., Rokeach L., Drèze P., Garcia L., Couturier M. Mini-F encoded proteins: identification of a new 10.5 kilodalton species. EMBO J. 1983;2(11):1853–1861. doi: 10.1002/j.1460-2075.1983.tb01671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenlaub R. Mutants of the mini-F plasmid pML31 thermosensitive in replication. J Bacteriol. 1979 May;138(2):559–566. doi: 10.1128/jb.138.2.559-566.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. W., Jr, Chao L. Altered stability and integration frequency of a F' factor in RNA polymerase mutants of Escherichia coli. Mol Gen Genet. 1981;182(1):12–18. doi: 10.1007/BF00422760. [DOI] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoui H., Bex F., Drèze P., Couturier M. Ham22, a mini-F mutation which is lethal to host cell and promotes recA-dependent induction of lambdoid prophage. EMBO J. 1983;2(11):1863–1868. doi: 10.1002/j.1460-2075.1983.tb01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C., Trawick J. Identification and characterization of a second copy number control gene in mini-F plasmids. Mol Gen Genet. 1983;192(3):408–415. doi: 10.1007/BF00392183. [DOI] [PubMed] [Google Scholar]
- Komai N., Nishizawa T., Hayakawa Y., Murotsu T., Matsubara K. Detection and mapping of six miniF-encoded proteins by cloning analysis of dissected miniF segments. Mol Gen Genet. 1982;186(2):193–203. doi: 10.1007/BF00331850. [DOI] [PubMed] [Google Scholar]
- Kopecko D. J., Brevet J., Cohen S. N. Involvement of multiple translocating DNA segments and recombinational hotspots in the structural evolution of bacterial plasmids. J Mol Biol. 1976 Dec;108(2):333–360. doi: 10.1016/s0022-2836(76)80124-6. [DOI] [PubMed] [Google Scholar]
- Lane H. E. Replication and incompatibility of F and plasmids in the IncFI Group. Plasmid. 1981 Jan;5(1):100–126. doi: 10.1016/0147-619x(81)90079-2. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Method for the isolation of the replication region of a bacterial replicon: construction of a mini-F'kn plasmid. J Bacteriol. 1976 Aug;127(2):982–987. doi: 10.1128/jb.127.2.982-987.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki S., Kuribayashi M., Miki T., Horiuchi T. An amber replication mutant of F plasmid mapped in the minimal replication region. Mol Gen Genet. 1983;191(2):231–237. doi: 10.1007/BF00334819. [DOI] [PubMed] [Google Scholar]
- Manis J. J., Kline B. C. F plasmid incompatibility and copy number genes: their map locations and interactions. Plasmid. 1978 Sep;1(4):492–507. doi: 10.1016/0147-619x(78)90007-0. [DOI] [PubMed] [Google Scholar]
- Miki T., Chang Z. T., Horiuchi T. Control of cell division by sex factor F in Escherichia coli. II. Identification of genes for inhibitor protein and trigger protein on the 42.84-43.6 F segment. J Mol Biol. 1984 Apr 25;174(4):627–646. doi: 10.1016/0022-2836(84)90087-1. [DOI] [PubMed] [Google Scholar]
- Miki T., Yoshioka K., Horiuchi T. Control of cell division by sex factor F in Escherichia coli. I. The 42.84-43.6 F segment couples cell division of the host bacteria with replication of plasmid DNA. J Mol Biol. 1984 Apr 25;174(4):605–625. doi: 10.1016/0022-2836(84)90086-x. [DOI] [PubMed] [Google Scholar]
- Murotsu T., Matsubara K., Sugisaki H., Takanami M. Nine unique repeating sequences in a region essential for replication and incompatibility of the mini-F plasmid. Gene. 1981 Nov;15(2-3):257–271. doi: 10.1016/0378-1119(81)90135-9. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Mini-F plasmid genes that couple host cell division to plasmid proliferation. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4784–4788. doi: 10.1073/pnas.80.15.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983 Feb;32(2):351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- Seelke R. W., Kline B. C., Trawick J. D., Ritts G. D. Genetic studies of F plasmid maintenance genes involved in copy number control, incompatability, and partitioning. Plasmid. 1982 Mar;7(2):163–179. doi: 10.1016/0147-619x(82)90075-0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Thompson R., Broda P. DNA polymerase 3 and the replication of F and ColVBtrp in Escherichia coli K-12. Mol Gen Genet. 1973 Dec 31;127(3):255–258. doi: 10.1007/BF00333765. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolun A., Helinski D. R. Direct repeats of the F plasmid incC region express F incompatibility. Cell. 1981 Jun;24(3):687–694. doi: 10.1016/0092-8674(81)90095-7. [DOI] [PubMed] [Google Scholar]
- Tsutsui H., Fujiyama A., Murotsu T., Matsubara K. Role of nine repeating sequences of the mini-F genome for expression of F-specific incompatibility phenotype and copy number control. J Bacteriol. 1983 Jul;155(1):337–344. doi: 10.1128/jb.155.1.337-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. Plasmid incompatibility and control of replication: copy mutants of the R-factor R1 in Escherichia coli K-12. J Bacteriol. 1975 Nov;124(2):641–649. doi: 10.1128/jb.124.2.641-649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Van Brunt J., Waggoner B. T., Pato M. L. Re-examination of F plasmid replication in a dnaC mutant of Escherichia coli. Mol Gen Genet. 1977 Feb 15;150(3):285–292. doi: 10.1007/BF00268127. [DOI] [PubMed] [Google Scholar]
- Wada C., Hiraga S., Yura T. A mutant of Escherichia coli incapable of supporting vegetative replication of F-like plasmids. J Mol Biol. 1976 Nov;108(1):25–41. doi: 10.1016/s0022-2836(76)80092-7. [DOI] [PubMed] [Google Scholar]
- Wada C., Yura T. Escherichia coli mutants incapable of supporting replication of F-like plasmids at high temperature: isolation and characterization of mafA and mafB mutants. J Bacteriol. 1979 Dec;140(3):864–873. doi: 10.1128/jb.140.3.864-873.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada C., Yura T., Hiraga S. Replication of Fpoh+ plasmid in mafA mutants of Escherichia coli defective in plasmid maintenance. Mol Gen Genet. 1977 Apr 29;152(3):211–217. doi: 10.1007/BF00268820. [DOI] [PubMed] [Google Scholar]
- Wada C., Yura T. Inhibition of initiation of mini-F plasmid replication in temperature-sensitive mafA mutants of Escherichia coli K-12. Plasmid. 1982 Nov;8(3):287–298. doi: 10.1016/0147-619x(82)90066-x. [DOI] [PubMed] [Google Scholar]
- Watson L. A., Phua S. H., Bergquist P. L., Lane H. E. An Mr 29000 protein is essential for mini-F maintenance in E. coli. Gene. 1982 Sep;19(2):173–178. doi: 10.1016/0378-1119(82)90003-8. [DOI] [PubMed] [Google Scholar]
- Wechsler J., Kline B. C. Mutation and identification of the F plasmid locus determining resistance to acridine orange curing. Plasmid. 1980 Nov;4(3):276–280. doi: 10.1016/0147-619x(80)90066-9. [DOI] [PubMed] [Google Scholar]
- Wehlmann H., Eichenlaub R. Plasmid mini-F encoded proteins. Mol Gen Genet. 1980;180(1):205–211. doi: 10.1007/BF00267371. [DOI] [PubMed] [Google Scholar]


