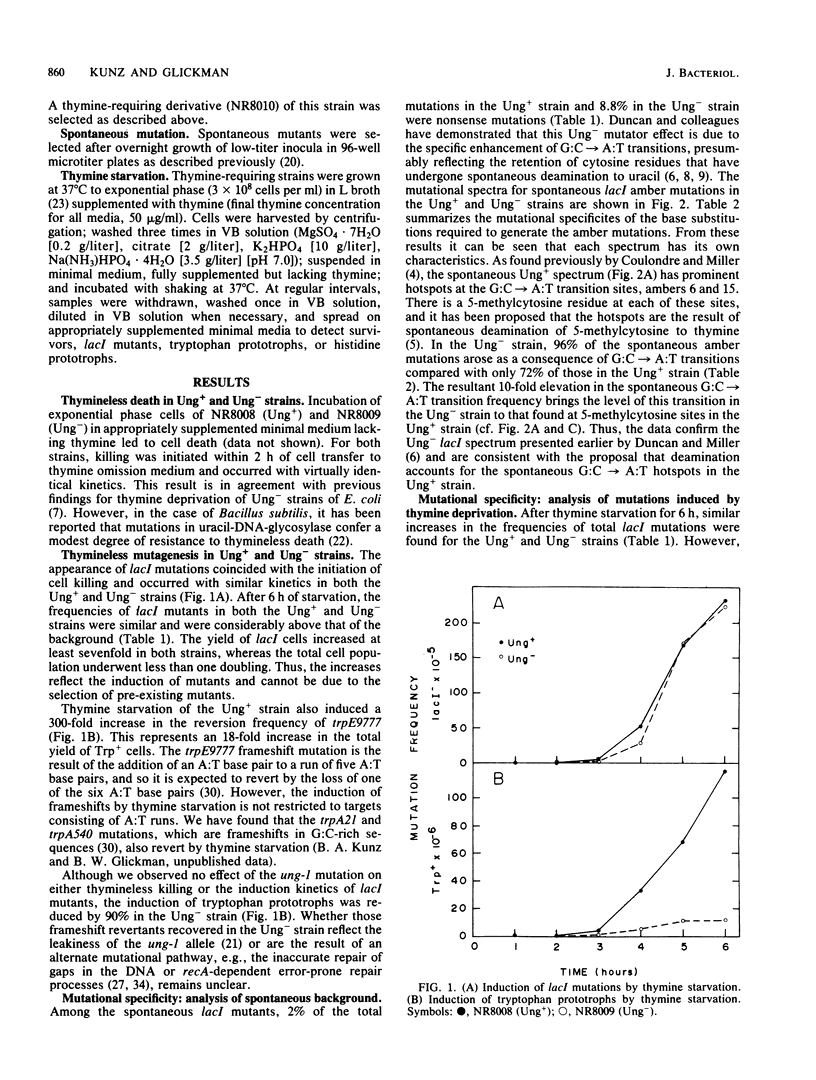
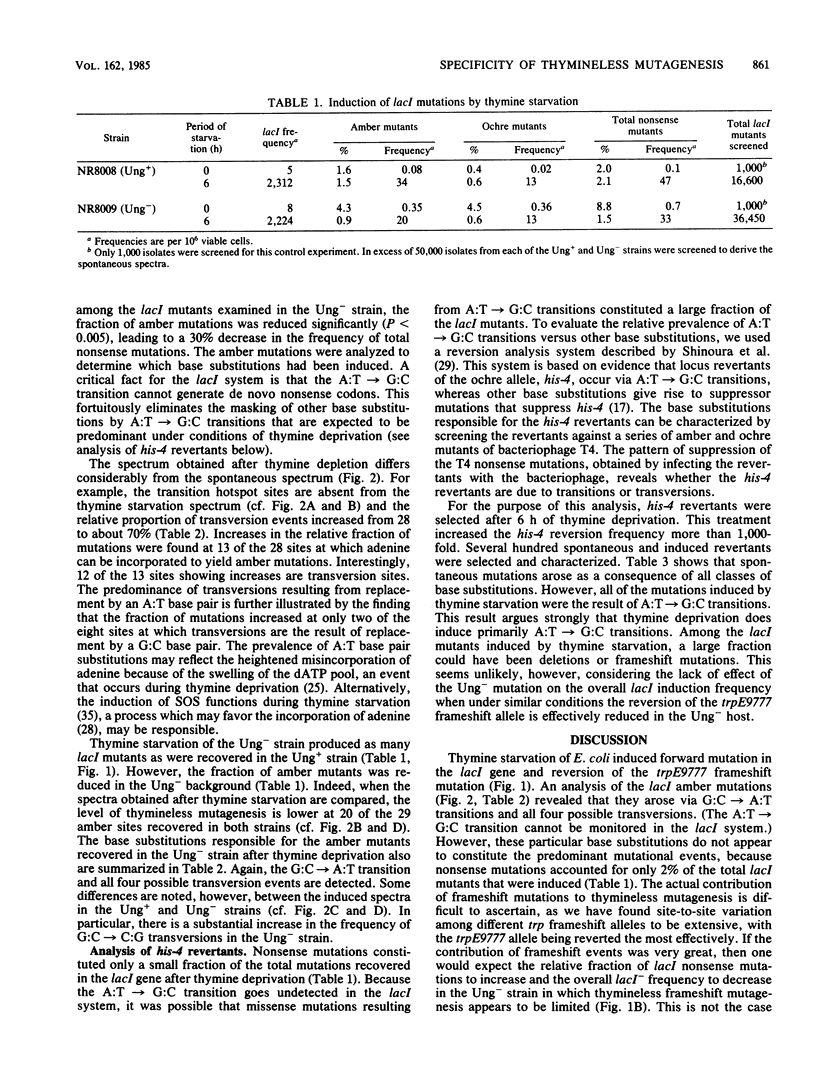
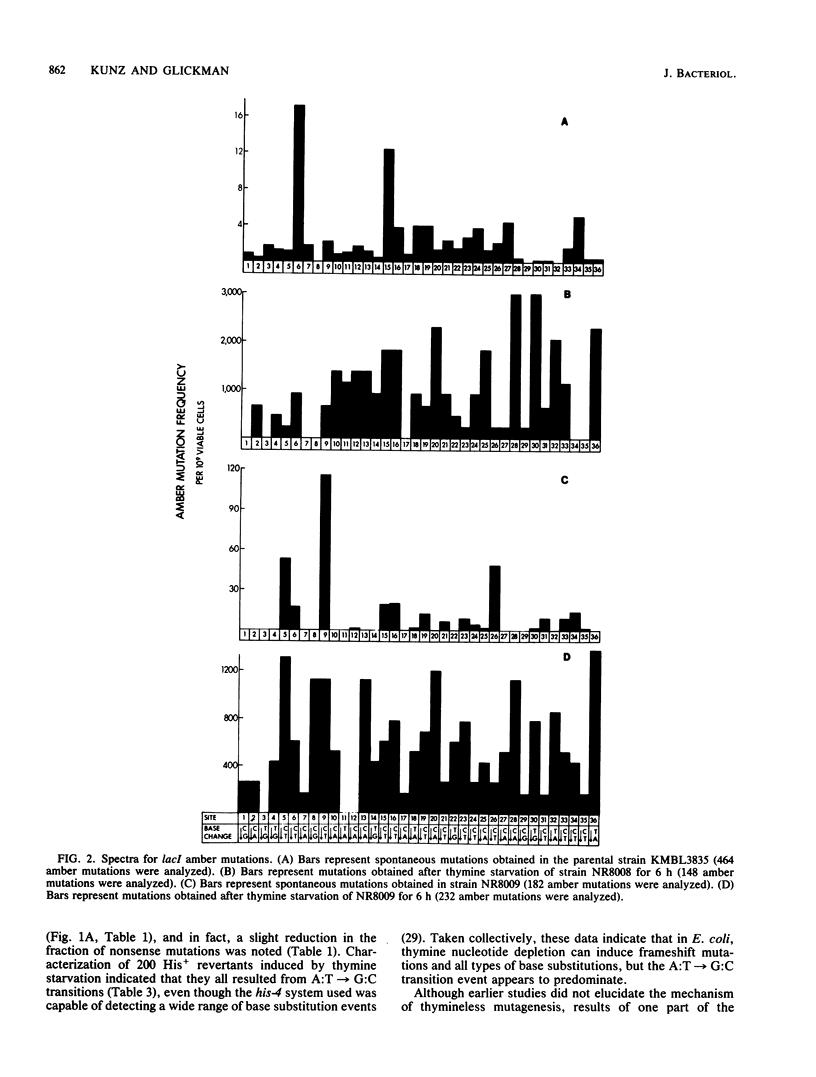
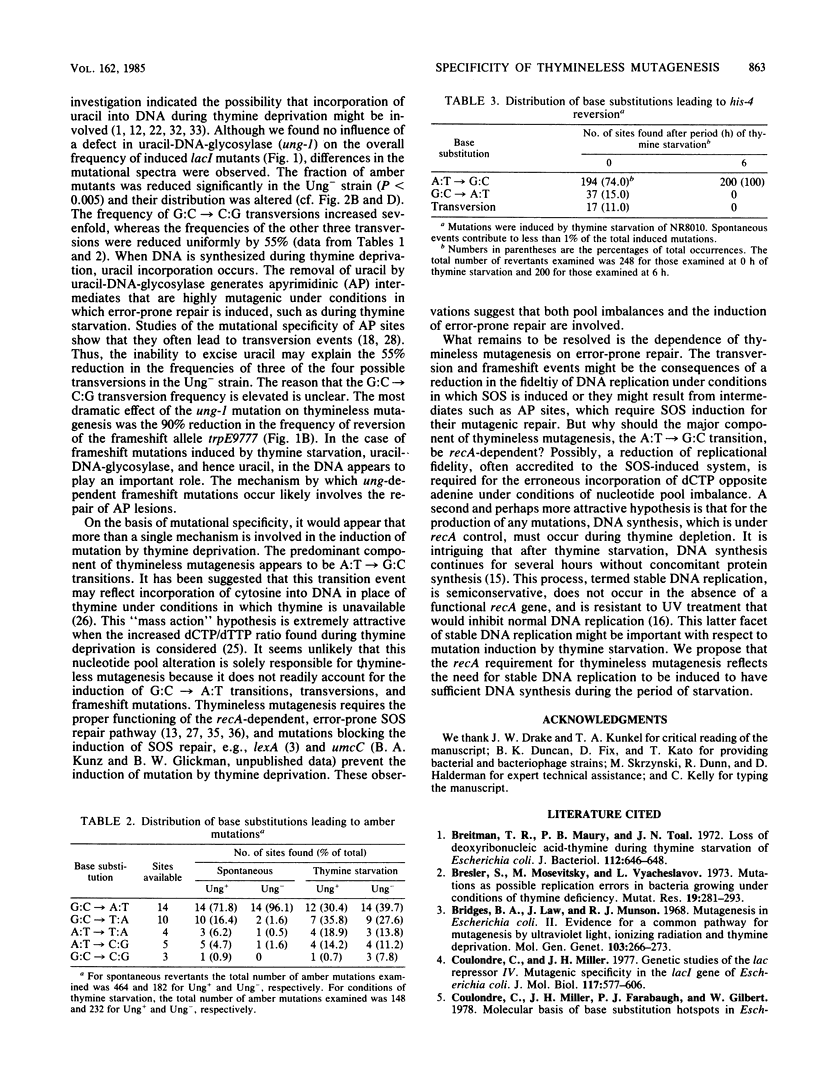
Abstract
To probe the mechanisms of mutagenesis induced by thymine starvation, we examined the mutational specificity of this treatment in strains of Escherichia coli that are wild type (Ung+) or deficient in uracil-DNA-glycosylase (Ung-). An analysis of Ung+ his-4 (ochre) revertants revealed that the majority of induced DNA base substitution events were A:T----G:C transitions. However, characterization of lacI nonsense mutations induced by thymine starvation demonstrated that G:C----A:T transitions and all four possible transversions also occurred. In addition, thymineless episodes led to reversion of the trpE9777 frameshift allele. Although the defect in uracil-DNA-glycosylase did not appear to affect the frequency of total mutations induced in lacI by thymine deprivation, the frequency of nonsense mutations was reduced by 30%, and the spectrum of nonsense mutations was altered. Furthermore, the reversion of trpE9777 was decreased by 90% in the Ung- strain. These findings demonstrate that in E. coli, thymine starvation can induce frameshift mutations and all types of base substitutions. The analysis of mutational specificity indicates that more than a single mechanism is involved in the induction of mutation by thymine depletion. We suggest that deoxyribonucleoside triphosphate pool imbalances, the removal of uracil incorporated into DNA during thymine starvation, and the induction of recA-dependent DNA repair functions all may play a role in thymineless mutagenesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breitman T. R., Maury P. B., Toal J. N. Loss of deoxyribonucleic acid-thymine during thymine starvation of Escherichia coli. J Bacteriol. 1972 Oct;112(1):646–648. doi: 10.1128/jb.112.1.646-648.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresler S. E., Mosevitsky M. I., Vyacheslavov L. G. Mutations as possible replication errors in bacteria growing under conditions of thymine deficiency. Mutat Res. 1973 Sep;19(3):281–293. doi: 10.1016/0027-5107(73)90228-5. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Law J., Munson R. J. Mutagenesis in Escherichia coli. II. Evidence for a common pathway for mutagenesis by ultraviolet light, ionizing radiation and thymine deprivation. Mol Gen Genet. 1968;103(3):266–273. doi: 10.1007/BF00273698. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Duncan B. K., Miller J. H. Mutagenic deamination of cytosine residues in DNA. Nature. 1980 Oct 9;287(5782):560–561. doi: 10.1038/287560a0. [DOI] [PubMed] [Google Scholar]
- Duncan B. K., Rockstroh P. A., Warner H. R. Escherichia coli K-12 mutants deficient in uracil-DNA glycosylase. J Bacteriol. 1978 Jun;134(3):1039–1045. doi: 10.1128/jb.134.3.1039-1045.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan B. K., Weiss B. Specific mutator effects of ung (uracil-DNA glycosylase) mutations in Escherichia coli. J Bacteriol. 1982 Aug;151(2):750–755. doi: 10.1128/jb.151.2.750-755.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh P. J. Sequence of the lacI gene. Nature. 1978 Aug 24;274(5673):765–769. doi: 10.1038/274765a0. [DOI] [PubMed] [Google Scholar]
- Glickman B. W. Spontaneous mutagenesis in Escherichia coli strains lacking 6-methyladenine residues in their DNA: an altered mutational spectrum in dam- mutants. Mutat Res. 1979 Jul;61(2):153–162. doi: 10.1016/0027-5107(79)90122-2. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Holmes A. J., Eisenstark A. The mutagenic effect of thymine-starvation on Salmonella typhimurium. Mutat Res. 1968 Jan-Feb;5(1):15–21. doi: 10.1016/0027-5107(68)90076-6. [DOI] [PubMed] [Google Scholar]
- Kato T., Shinoura Y., Templin A., Clark A. J. Analysis of ultraviolet light-induced suppressor mutations in the strain of Escherichia coli K-12 AB1157: an implication for molecular mechanisms of UV mutagenesis. Mol Gen Genet. 1980;180(2):283–291. doi: 10.1007/BF00425840. [DOI] [PubMed] [Google Scholar]
- Kogoma T., Lark K. G. Characterization of the replication of Escherichia coli DNA in the absence of protein synthesis: stable DNA replication. J Mol Biol. 1975 May 15;94(2):243–256. doi: 10.1016/0022-2836(75)90081-9. [DOI] [PubMed] [Google Scholar]
- Kogoma T., Torrey T. A., Connaughton M. J. Induction of UV-resistant DNA replication in Escherichia coli: induced stable DNA replication as an SOS function. Mol Gen Genet. 1979 Oct 2;176(1):1–9. doi: 10.1007/BF00334288. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Mutational specificity of depurination. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1494–1498. doi: 10.1073/pnas.81.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz B. A. Genetic effects of deoxyribonucleotide pool imbalances. Environ Mutagen. 1982;4(6):695–725. doi: 10.1002/em.2860040609. [DOI] [PubMed] [Google Scholar]
- Kunz B. A., Glickman B. W. The infidelity of conjugal DNA transfer in Escherichia coli. Genetics. 1983 Nov;105(3):489–500. doi: 10.1093/genetics/105.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Makino F., Munakata N. Deoxyuridine residues in DNA of thymine-requiring Bacillus subtilis strains with defective N-glycosidase activity for uracil-containing DNA. J Bacteriol. 1978 Apr;134(1):24–29. doi: 10.1128/jb.134.1.24-29.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Coulondre C., Farabaugh P. J. Correlation of nonsense sites in the lacI gene with specific codons in the nucleotide sequence. Nature. 1978 Aug 24;274(5673):770–775. doi: 10.1038/274770a0. [DOI] [PubMed] [Google Scholar]
- Neuhard J., Munch-Petersen A. Studies on the acid-soluble nucleotide pool in thymine-requiring mutants of Escherichia coli during thymine starvation. II. Changes in the amounts of deoxycytidine triphosphate and deoxyadenosine triphosphate in Escherichia coli 15 T-A-U. Biochim Biophys Acta. 1966 Jan 18;114(1):61–71. doi: 10.1016/0005-2787(66)90253-x. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Kunkel T. A., Loeb L. A. Infidelity of DNA synthesis associated with bypass of apurinic sites. Proc Natl Acad Sci U S A. 1983 Jan;80(2):487–491. doi: 10.1073/pnas.80.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Green R. R., Ripley L. S., Drake J. W. Thymineless mutagenesis in bacteriophage T4. Genetics. 1973 Jul;74(3):393–403. doi: 10.1093/genetics/74.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanoi F., Okazaki T. Uracil incorporation into nascent DNA of thymine-requiring mutant of Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1978 May;75(5):2195–2199. doi: 10.1073/pnas.75.5.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Duncan B. K., Garrett C., Neuhard J. Synthesis and metabolism of uracil-containing deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1981 Feb;145(2):687–695. doi: 10.1128/jb.145.2.687-695.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M., Wermundsen I. E. Targeted and untargeted mutagenesis by various inducers of SOS functions in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):881–886. doi: 10.1101/sqb.1979.043.01.095. [DOI] [PubMed] [Google Scholar]


