Abstract
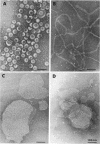
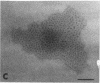
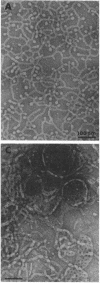
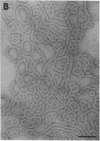
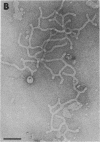
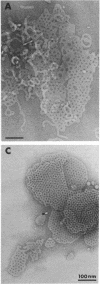
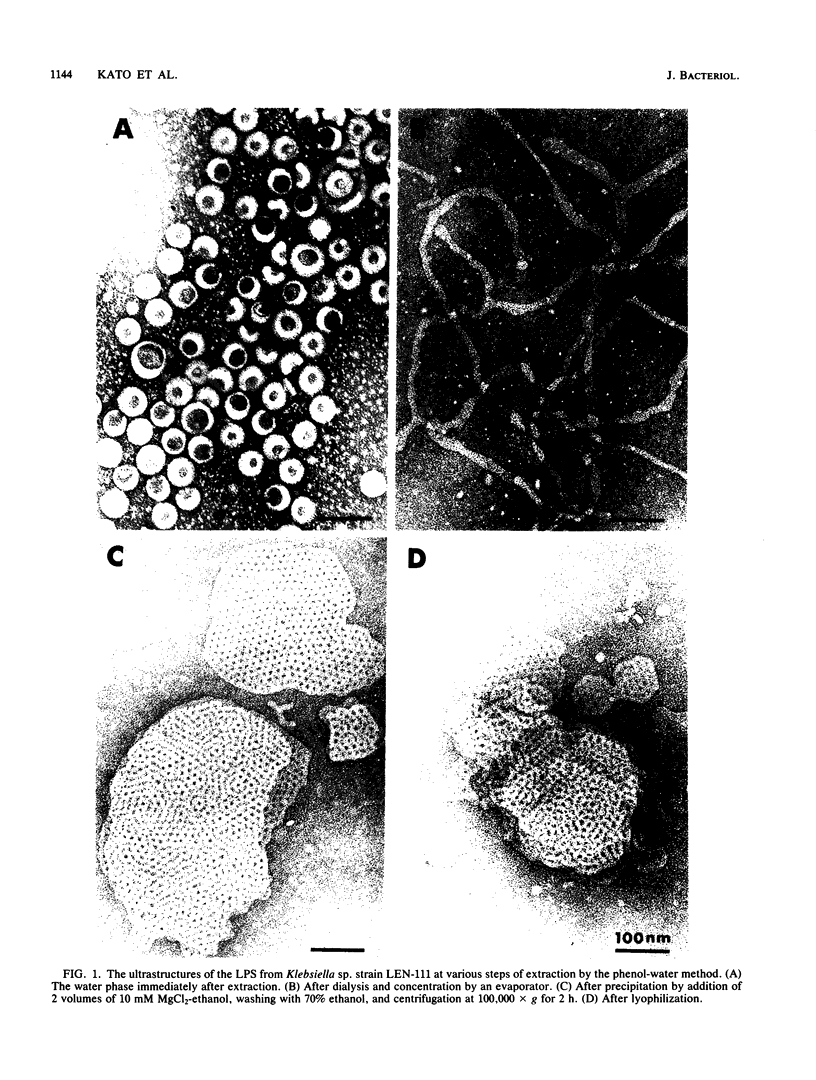
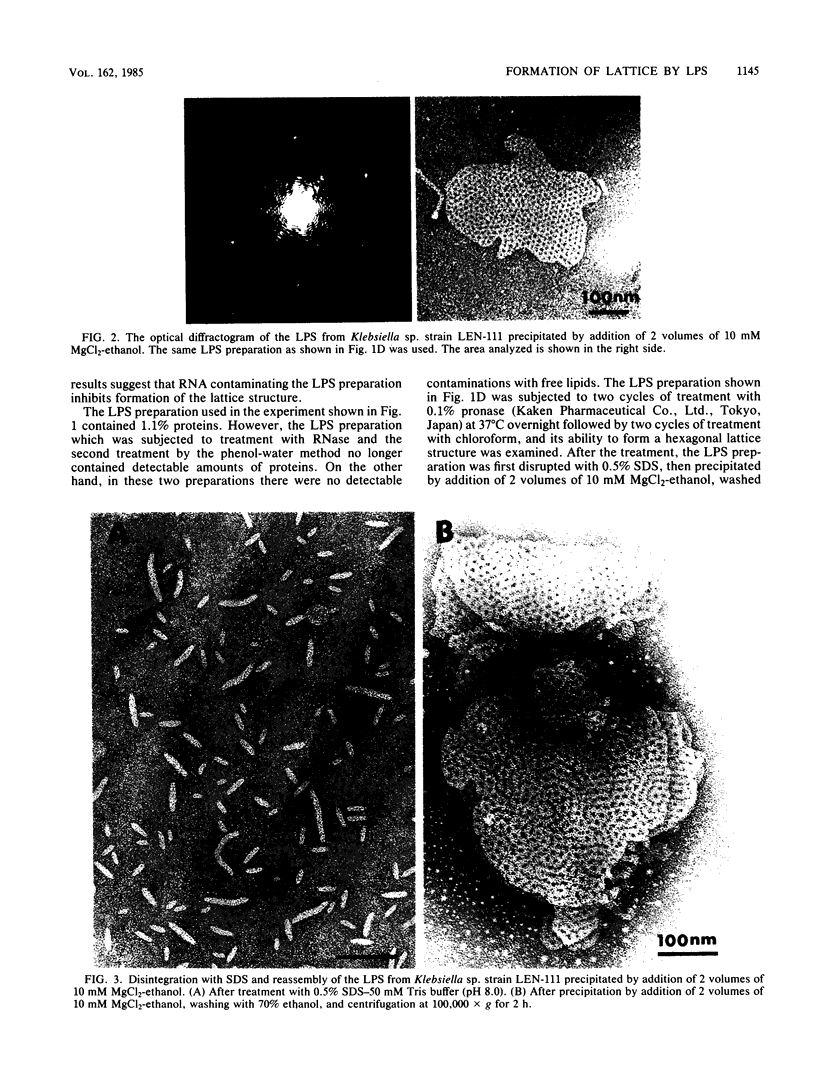
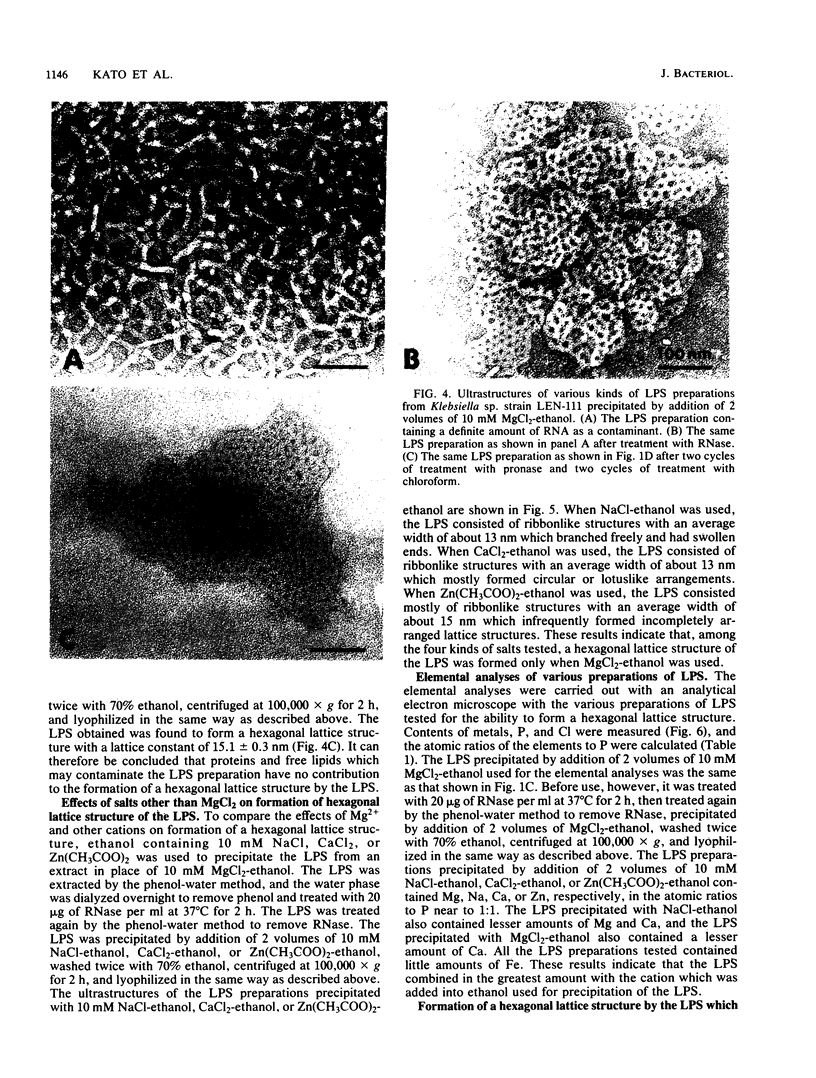
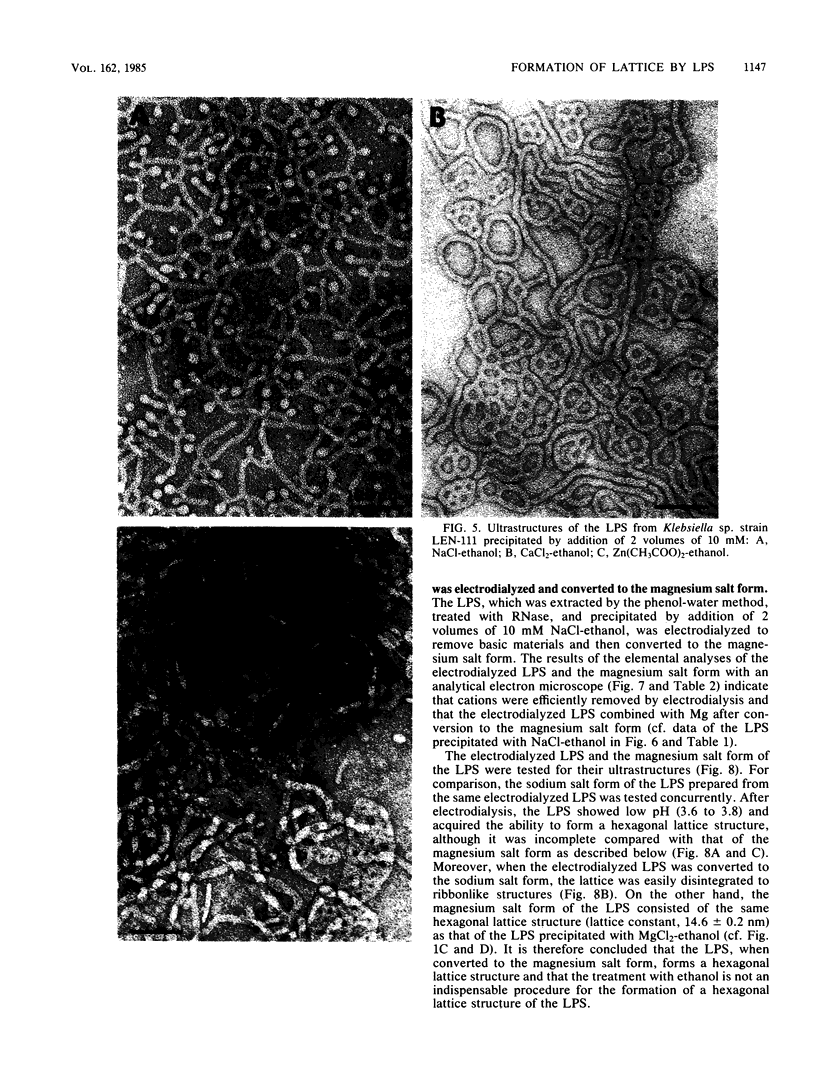
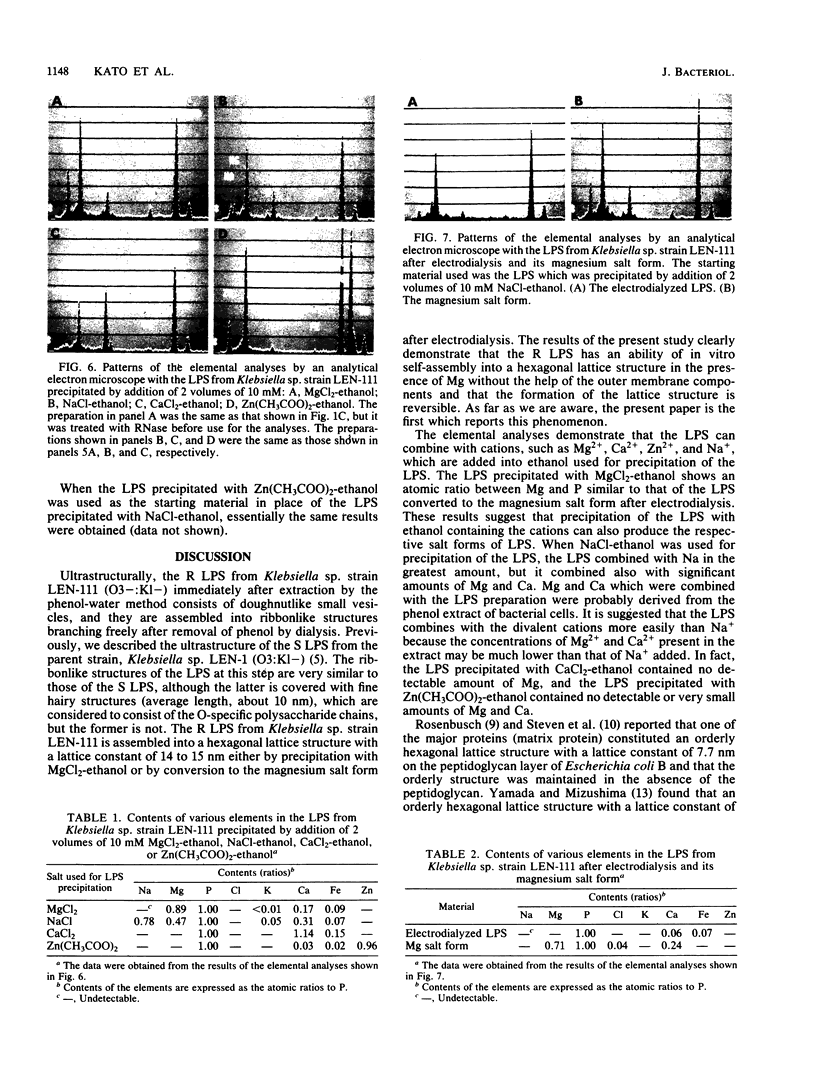
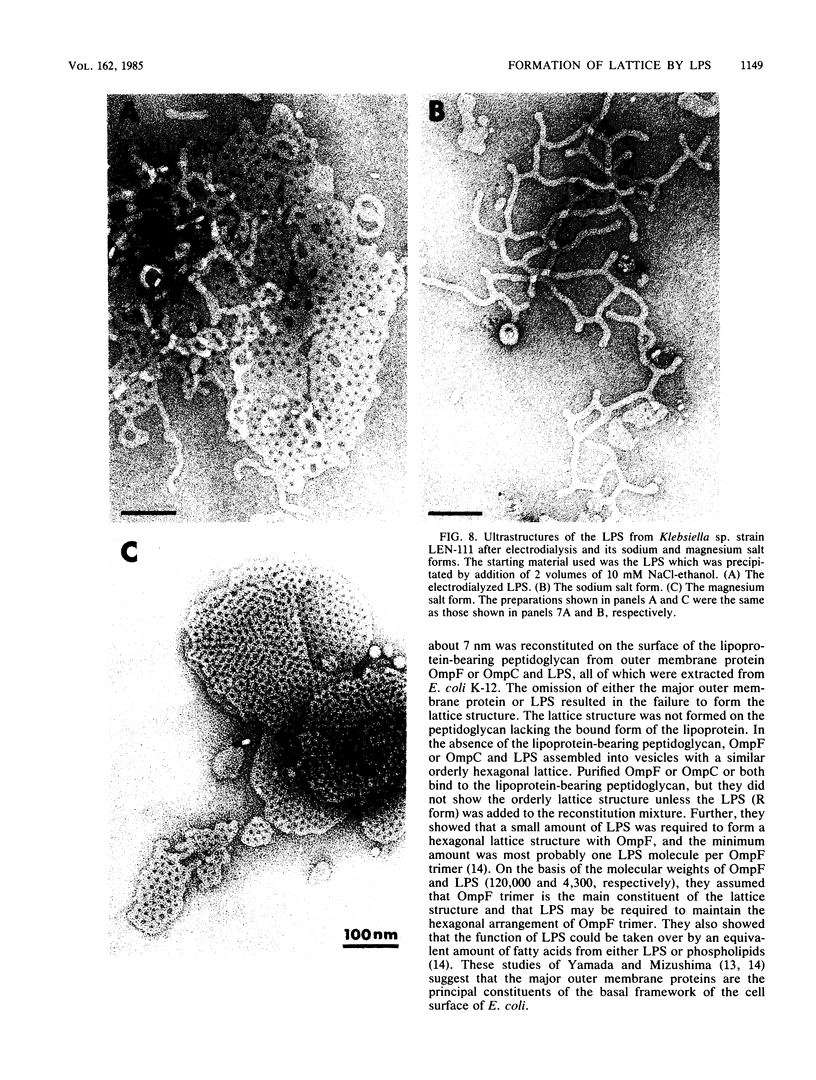
We extracted an R-form lipopolysaccharide (LPS) by the phenol-water method from Klebsiella sp. strain LEN-111 (O3-:KI-) and followed the changes in ultrastructure of the LPS during the extraction procedure. When the LPS was obtained from the water phase of an extract by addition of 2 volumes of 10 mM MgCI2-ethanol, it consisted of membrane pieces with a hexagonal lattice structure with a lattice constant of 14 to 15 nm. The lattice structure of the LPS was disrupted into short rods with sodium dodecyl sulfate, but the same hexagonal lattice structure was again formed by precipitation with 2 volumes of 10 mM MgCI2-ethanol. The LPS preparation after two cycles of treatment by the phenol-water method, which contained no detectable amounts of proteins, kept an unaltered ability to form the hexagonal lattice structure. Extensive treatment with pronase and extraction with chloroform did not impair the ability of the LPS preparation to form the lattice structure. When the other salts, NaCI, CaCI2 or Zn(CH3COO)2, were used for precipitation of the LPS with ethanol in place of MgCI2, the LPS did not form the hexagonal lattice structure. However, if the LPS precipitated with NaCI-ethanol was converted to the magnesium salt form after it was electrodialyzed, it formed the same hexagonal lattice structure as the LPS precipitated with MgCI2-ethanol. From these results, it was concluded that the R-form LPS has the ability of in vitro self-assembly into a hexagonal lattice structure in the presence of Mg2+ without the help of other components such as proteins and free lipids from outer membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curvall M., Lindberg B., Lönngren J., Nimmich W. Structural studies on the Klebsiella O group 3 lipopolysaccharide. Acta Chem Scand. 1973;27(7):2645–2649. doi: 10.3891/acta.chem.scand.27-2645. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Voter W. A., Leonard K. Image reconstruction in electron microscopy: enhancement of periodic structure by optical filtering. Methods Enzymol. 1978;49:39–63. doi: 10.1016/s0076-6879(78)49006-8. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Ohta M., Mori M., Nakashima I., Kato N. The Klebsiella O3 lipopolysaccharide isolated from culture fluid: structure of the polysaccharide moiety. Microbiol Immunol. 1983;27(8):683–694. doi: 10.1111/j.1348-0421.1983.tb00631.x. [DOI] [PubMed] [Google Scholar]
- Kato N., Ohta M., Kido N., Naito S., Kuno T. Ultrastructure of Klebsiella O3 lipopolysaccharide isolated from culture supernatant: comparison with other lipopolysaccharides. Microbiol Immunol. 1984;28(5):545–557. doi: 10.1111/j.1348-0421.1984.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Kato N., Ohta M., Kido N., Naito S., Kuno T. Ultrastructure of Klebsiella O3 lipopolysaccharide isolated from culture supernatant: structure of various uniform salt forms. Microbiol Immunol. 1984;28(5):559–567. doi: 10.1111/j.1348-0421.1984.tb00708.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ohta M., Mori M., Hasegawa T., Nagase F., Nakashima I., Naito S., Kato N. Further Studies of the polysaccharide of Klebsiella pneumoniae possessing strong adjuvanticity. I. Production of the adjuvant polysaccharide by noncapsulated mutant. Microbiol Immunol. 1981;25(9):939–948. doi: 10.1111/j.1348-0421.1981.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Steven A. C., Heggeler B., Müller R., Kistler J., Rosenbusch J. P. Ultrastructure of a periodic protein layer in the outer membrane of Escherichia coli. J Cell Biol. 1977 Feb;72(2):292–301. doi: 10.1083/jcb.72.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Interaction between major outer membrane protein (O-8) and lipopolysaccharide in Escherichia coli K12. Eur J Biochem. 1980 Jan;103(1):209–218. doi: 10.1111/j.1432-1033.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Reconstitution of an ordered structure from major outer membrane constituents and the lipoprotein-bearing peptidoglycan sacculus of Escherichia coli. J Bacteriol. 1978 Sep;135(3):1024–1031. doi: 10.1128/jb.135.3.1024-1031.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]