Abstract
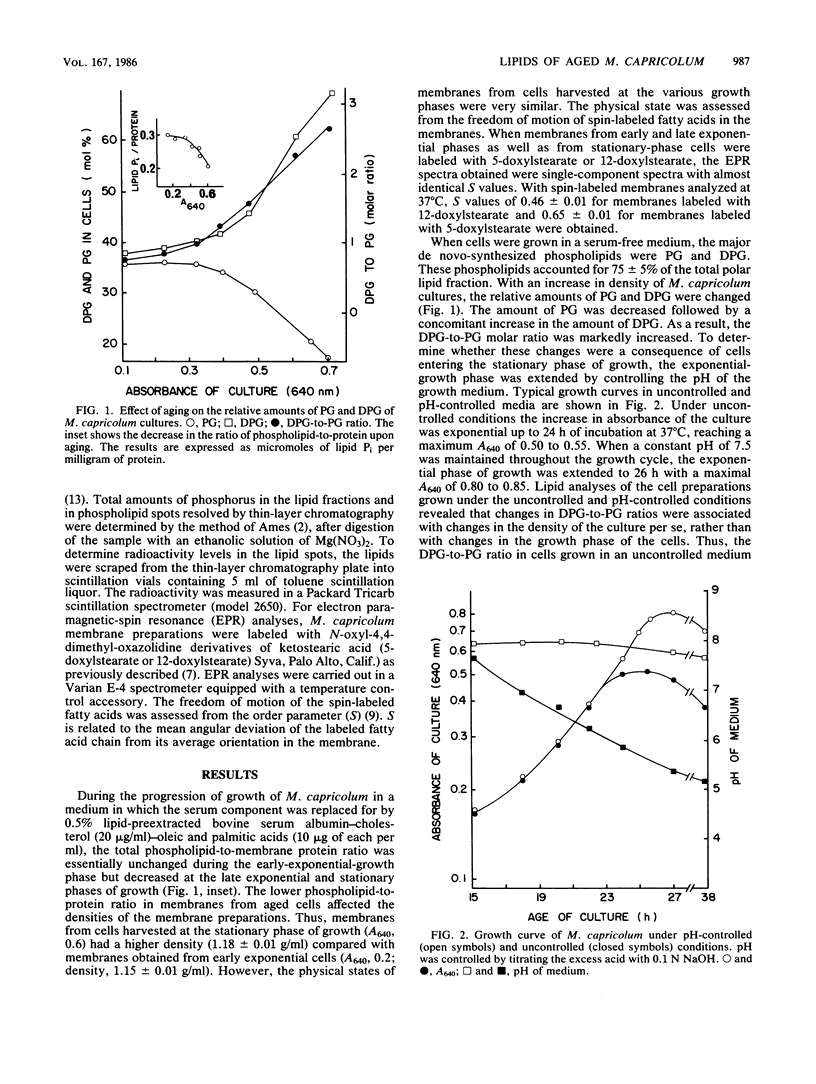
During the progression of Mycoplasma capricolum cultures from the early exponential to the stationary phase of growth, a decrease in the phospholipid-to-protein ratio and increases in both the unsaturated-to-saturated fatty acid ratio and the diphosphatidylglycerol (DPG)-to-phosphatidylglycerol (PG) ratio were found. The freedom of motion of spin-labeled fatty acids incorporated into the membrane remained unchanged throughout the growth cycle. The increase in DPG was almost stoichiometric with the decrease in PG. Furthermore, exogenous PG added to the medium was incorporated by the cells and partially converted to DPG. The DPG that was accumulated upon aging was always more unsaturated than the PG. This accumulation was enhanced in palmitic acid-poor media, but was inhibited even in aged cells when the cells were grown in palmitic acid-rich media, suggesting that the accumulation of DPG upon aging was associated with changes in the fatty acid composition of membrane lipids rather than with the transition of the cells from the exponential- to stationary-growth phase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar A., Rottem S., Kahane I., Razin S. Characterization of the mycoplasma membrane proteins. VI. Composition and disposition of proteins in membranes from aging Mycoplasma hominis cultures. Biochim Biophys Acta. 1976 Mar 5;426(2):258–270. doi: 10.1016/0005-2736(76)90336-9. [DOI] [PubMed] [Google Scholar]
- Audet A., Cole R., Proulx P. Polyglycerophosphatide metabolism in Escherichia coli. Biochim Biophys Acta. 1975 Mar 24;380(3):414–420. doi: 10.1016/0005-2760(75)90109-5. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Dahl J. S., Dahl C. E., Bloch K. Sterols in membranes: growth characteristics and membrane properties of Mycoplasma capricolum cultured on cholesterol and lanosterol. Biochemistry. 1980 Apr 1;19(7):1467–1472. doi: 10.1021/bi00548a032. [DOI] [PubMed] [Google Scholar]
- Davis P. J., Katznel A., Razin S., Rottem S. Spiroplasma membrane lipids. J Bacteriol. 1985 Jan;161(1):118–122. doi: 10.1128/jb.161.1.118-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney B. J. Fatty acid chain flexibility in the membranes of normal and transformed fibroblasts. Proc Natl Acad Sci U S A. 1975 Feb;72(2):664–668. doi: 10.1073/pnas.72.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine H. Bacterial membranes and lipid packing theory. J Lipid Res. 1984 Dec 15;25(13):1501–1507. [PubMed] [Google Scholar]
- Gross Z., Rottem S., Bittman R. Phospholipid interconversions in Mycoplasma capricolum. Eur J Biochem. 1982 Feb;122(1):169–174. doi: 10.1111/j.1432-1033.1982.tb05863.x. [DOI] [PubMed] [Google Scholar]
- Hirschberg C. B., Kennedy E. P. Mechanism of the enzymatic synthesis of cardiolipin in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Mar;69(3):648–651. doi: 10.1073/pnas.69.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Michel G., Di Savino D., Starka J. Phospholipid composition and phenotypic correction of an envC division mutant of Escherichia coli. J Bacteriol. 1977 Jan;129(1):145–150. doi: 10.1128/jb.129.1.145-150.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panos C., Rottem S. Incorporation and elongation of fatty acid isomers by Mycoplasma laidlawii A. Biochemistry. 1970 Jan 20;9(2):407–412. doi: 10.1021/bi00804a030. [DOI] [PubMed] [Google Scholar]
- Plackett P. The glycerolipids of Mycoplasma mycoides. Biochemistry. 1967 Sep;6(9):2746–2754. doi: 10.1021/bi00861a015. [DOI] [PubMed] [Google Scholar]
- Razin S. Cholesterol uptake is dependent on membrane fluidity in mycoplasmas. Biochim Biophys Acta. 1978 Nov 16;513(3):401–404. doi: 10.1016/0005-2736(78)90208-0. [DOI] [PubMed] [Google Scholar]
- Razin S., Kutner S., Efrati H., Rottem S. Phospholipid and cholesterol uptake by Mycoplasma cells and membranes. Biochim Biophys Acta. 1980 Jun 6;598(3):628–640. doi: 10.1016/0005-2736(80)90042-5. [DOI] [PubMed] [Google Scholar]
- Rigomier D., Lacombe C., Lubochinsky B. Cardiolipin metabolism in growing and sporulating Bacillus subtilis. FEBS Lett. 1978 May 1;89(1):131–135. doi: 10.1016/0014-5793(78)80538-9. [DOI] [PubMed] [Google Scholar]
- Rottem S., Greenberg A. S. Changes in composition, biosynthesis, and physical state of membrane lipids occurring upon aging of Mycoplasma hominis cultures. J Bacteriol. 1975 Feb;121(2):631–639. doi: 10.1128/jb.121.2.631-639.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S. Membrane lipids of mycoplasmas. Biochim Biophys Acta. 1980 May 27;604(1):65–90. doi: 10.1016/0005-2736(80)90585-4. [DOI] [PubMed] [Google Scholar]
- Rottem S., Samuni A. Effect of proteins on the motion of spin-labeled fatty acids in mycoplasma membranes. Biochim Biophys Acta. 1973 Feb 27;298(1):32–38. doi: 10.1016/0005-2736(73)90006-0. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J. Lipidic intramembranous particles. Biochim Biophys Acta. 1984 Jan 27;779(1):43–63. doi: 10.1016/0304-4157(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Wieslander A., Christiansson A., Rilfors L., Lindblom G. Lipid bilayer stability in membranes. Regulation of lipid composition in Acholeplasma laidlawii as governed by molecular shape. Biochemistry. 1980 Aug 5;19(16):3650–3655. doi: 10.1021/bi00557a002. [DOI] [PubMed] [Google Scholar]
- Wieslander A., Rilfors L. Qualitative and quantitative variations of membrane lipid species in Acholeplasma laidlawii A. Biochim Biophys Acta. 1977 Apr 18;466(2):336–346. doi: 10.1016/0005-2736(77)90229-2. [DOI] [PubMed] [Google Scholar]


