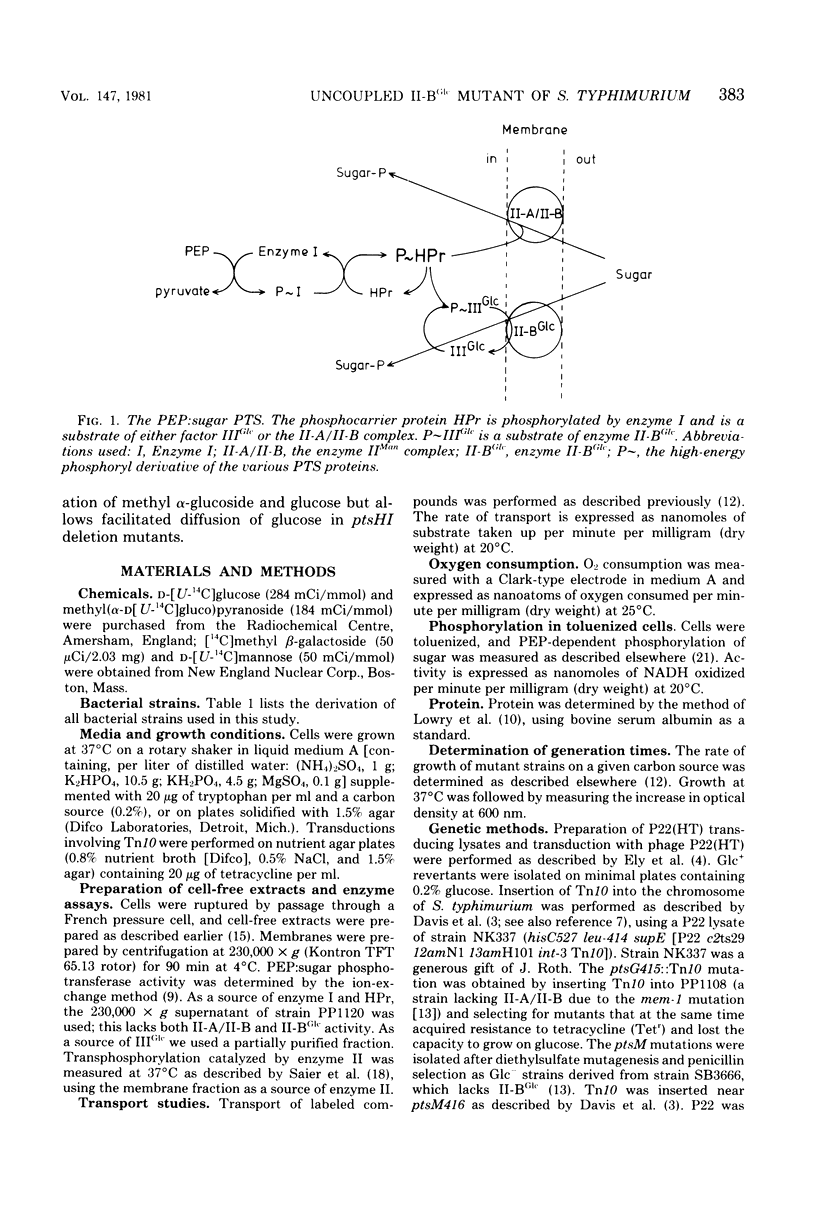
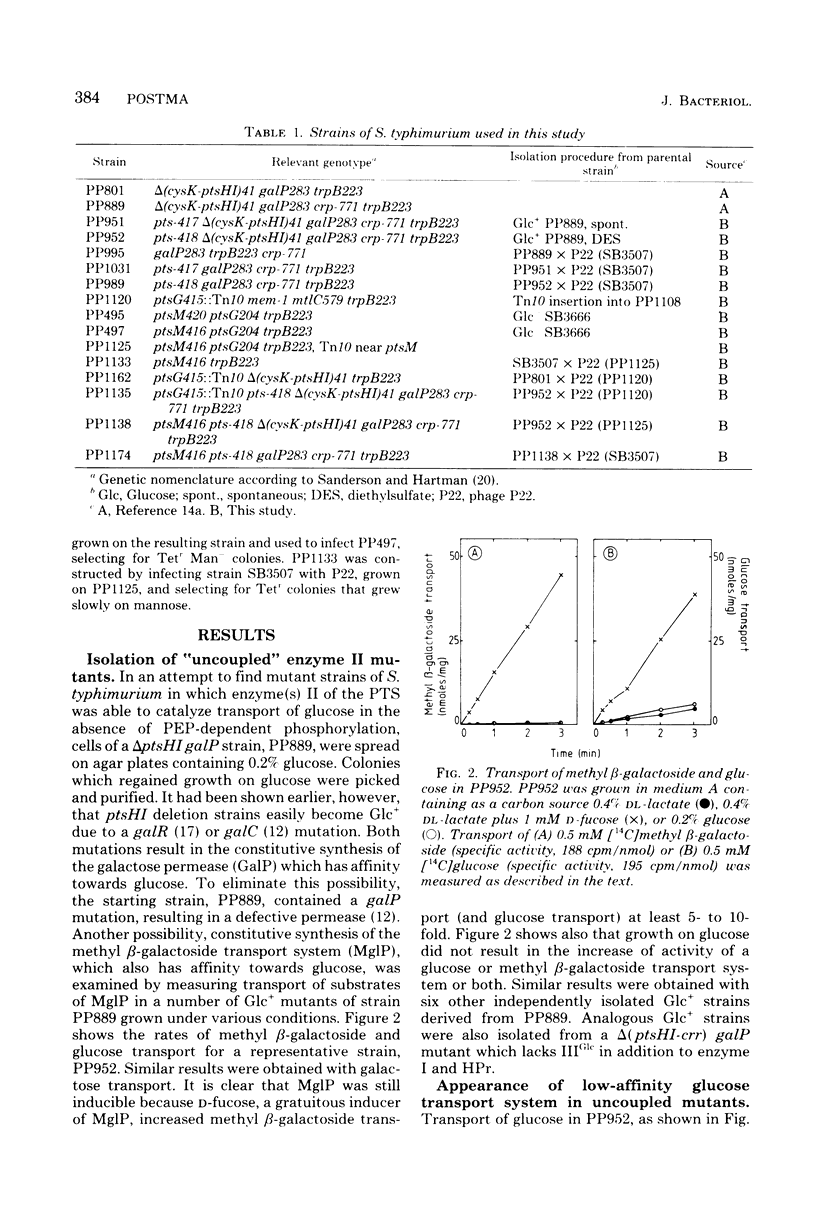
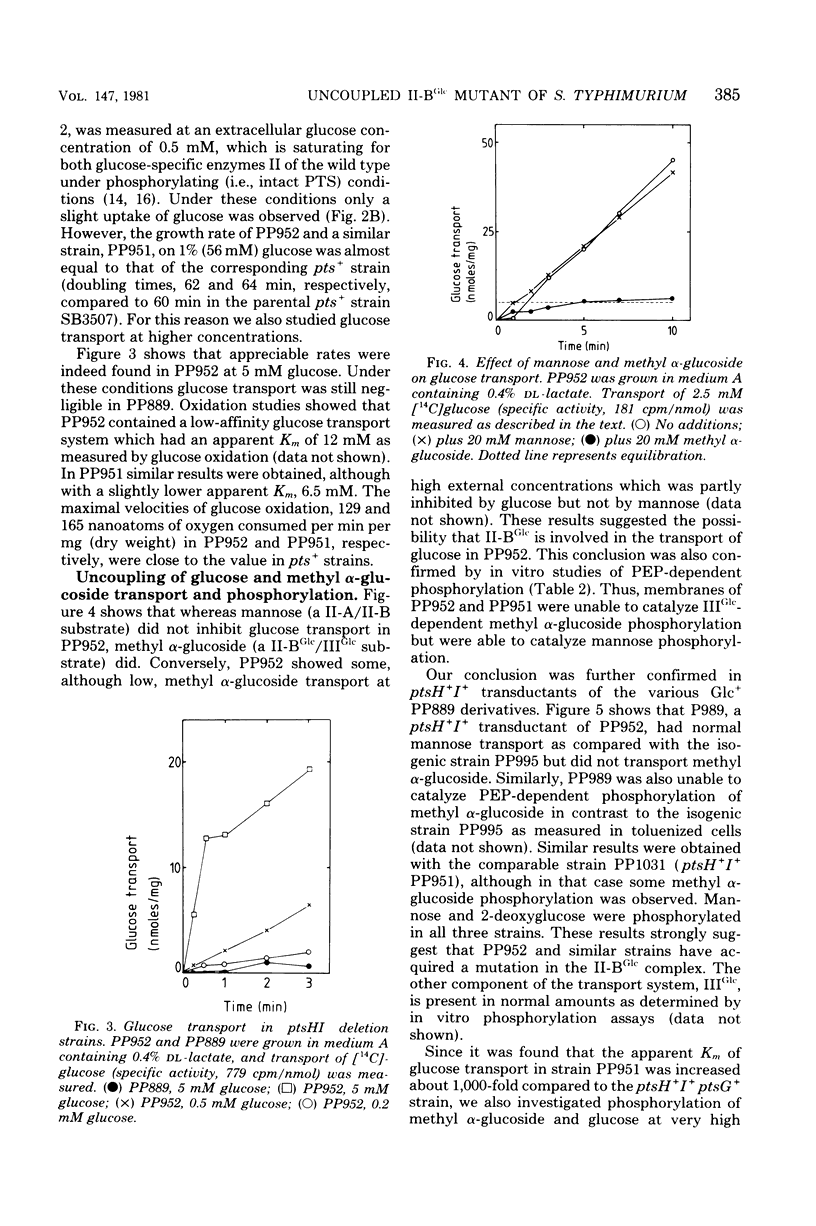
Abstract
Transport and phosphorylation of glucose via enzymes II-A/II-B and II-BGlc of the phosphoenolpyruvate:sugar phosphotransferase system are tightly coupled in Salmonella typhimurium. Mutant strains (pts) that lack the phosphorylating proteins of this system, enzyme I and HPr, are unable to transport or to grow on glucose. From ptsHI deletion strains of S. typhimurium, mutants were isolated that regained growth on and transport of glucose. Several lines of evidence suggest that these Glc+ mutants have an altered enzyme II-BGlc as follows. (i) Insertion of a ptsG::Tn10 mutation (resulting in a defective II-BGlc) abolished growth on and transport of glucose in these Glc+ strains. Introduction of a ptsM mutation, on the other hand, which abolishes II-A/II-B activity, had no effect. (ii) Methyl alpha-glucoside transport and phosphorylation (specific for II-BGlc) was lowered or absent in ptsH+,I+ transductants of these Glc+ strains. Transport and phosphorylation of other phosphoenolpyurate:sugar phosphotransferase system sugars were normal. (iii) Membranes isolated from these Glc+ mutants were unable to catalyze transphosphorylation of methyl alpha-glucoside by glucose 6-phosphate, but transphosphorylation of mannose by glucose 6-phosphate was normal. (iv) The mutation was in the ptsG gene or closely linked to it. We conclude that the altered enzyme II-BGlc has acquired the capacity to transport glucose in the absence of phosphoenolpyruvate:sugar phosphotransferase system-mediated phosphorylation. However, the affinity for glucose decreased at least 1,000-fold as compared to the wild-type strain. At the same time the mutated enzyme II-BGlc lost the ability to catalyze the phosphorylation of its substrates via IIIGlc.
Full text
PDF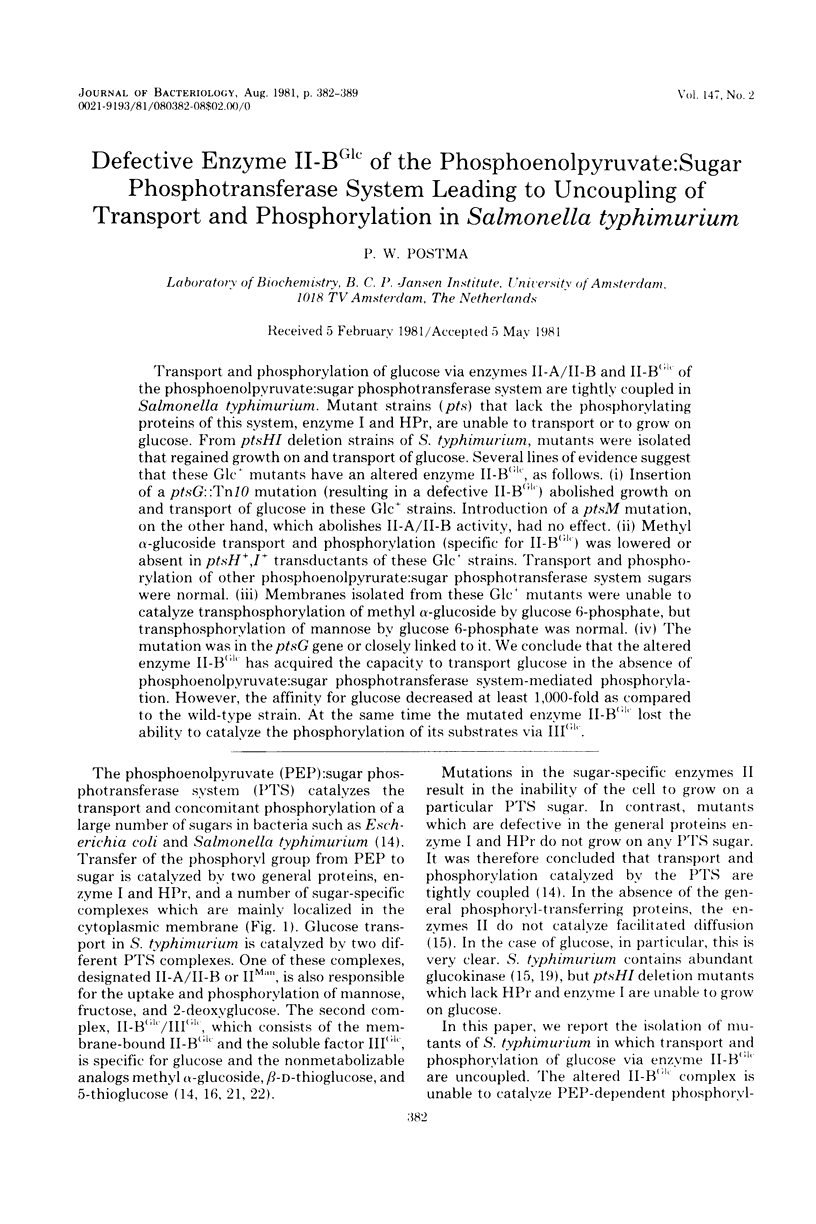



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourd G. I., Erlagaeva R. S., Bolshakova T. N., Gershanovitch V. N. Glucose catabolite repression in Escherichia coli K12 mutants defective in methyl-alpha-d-glucoside transport. Eur J Biochem. 1975 May 6;53(2):419–427. doi: 10.1111/j.1432-1033.1975.tb04082.x. [DOI] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlagaeva R. S., Bolshakova T. N., Shulgina M. V., Bourd G. I., Gershanovitch V. N. Glucose effect in tgl mutant of Escherichia col K12 defective in methyl-alpha-D-glucoside transport. Eur J Biochem. 1977 Jan 3;72(1):127–135. doi: 10.1111/j.1432-1033.1977.tb11232.x. [DOI] [PubMed] [Google Scholar]
- Gachelin G. Studies on the alpha-methylglucoside permease of Escherichia coli. A two-step mechanism for the accumulation of alpha-methylglucoside 6-phosphate. Eur J Biochem. 1970 Oct;16(2):342–357. doi: 10.1111/j.1432-1033.1970.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Hartman P. E. Some improved methods in P22 transduction. Genetics. 1974 Apr;76(4):625–631. doi: 10.1093/genetics/76.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Genetic control of glucose uptake by Escherichia coli. FEBS Lett. 1972 Feb 15;20(3):270–272. doi: 10.1016/0014-5793(72)80084-x. [DOI] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Melton T., Kundig W., Hartman P. E., Meadow N. 3-Deoxy-3-fluoro-D-glucose-resistant Salmonella typhimurium mutants defective in the phosphoenolpyruvate:glycose phosphotransferase system. J Bacteriol. 1976 Dec;128(3):794–800. doi: 10.1128/jb.128.3.794-800.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Cordaro J. C., Roseman S. Sugar transport. A pleiotropic membrane mutant of Salmonella typhimurium. J Biol Chem. 1977 Nov 10;252(21):7862–7876. [PubMed] [Google Scholar]
- Postma P. W. Galactose transport in Salmonella typhimurium. J Bacteriol. 1977 Feb;129(2):630–639. doi: 10.1128/jb.129.2.630-639.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Schuitema A., Kwa C. Regulation of methyl beta-galactoside permease activity in pts and crr mutants of Salmonella typhimurium. Mol Gen Genet. 1981;181(4):448–453. doi: 10.1007/BF00428734. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Stock J. B. Enzymes II of the phosphotransferase system do not catalyze sugar transport in the absence of phosphorylation. J Bacteriol. 1980 Feb;141(2):476–484. doi: 10.1128/jb.141.2.476-484.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Kinetic analyses of the sugar phosphate:sugar transphosphorylation reaction catalyzed by the glucose enzyme II complex of the bacterial phosphotransferase system. J Biol Chem. 1978 Nov 10;253(21):7595–7597. [PubMed] [Google Scholar]
- Saier M. H., Jr, Bromberg F. G., Roseman S. Characterization of constitutive galactose permease mutants in Salmonella typhimurium. J Bacteriol. 1973 Jan;113(1):512–514. doi: 10.1128/jb.113.1.512-514.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Mora W. K. Sugar phosphate: sugar transphosphorylation and exchange group translocation catalyzed by the enzyme 11 complexes of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1977 Dec 25;252(24):8899–8907. [PubMed] [Google Scholar]
- Saier M. H., Jr, Young W. S., 3rd, Roseman S. Utilization and transport of hexoses by mutant strains of Salmonella typhimurium lacking enzyme I of the phosphoenolpyruvate-dependent phosphotransferase system. J Biol Chem. 1971 Sep 25;246(18):5838–5840. [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte B. J., Postma P. W. Competition between two pathways for sugar uptake by the phosphoenolpyruvate-dependent sugar phosphotransferase system in Salmonella typhimurium. Eur J Biochem. 1981;114(1):51–58. doi: 10.1111/j.1432-1033.1981.tb06171.x. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Meadow N. D., Roseman S. Modified assay procedures for the phosphotransferase system in enteric bacteria. Anal Biochem. 1979 May;95(1):293–304. doi: 10.1016/0003-2697(79)90219-7. [DOI] [PubMed] [Google Scholar]
- West I. C., Wilson T. H. Galactoside transport dissociated from proton movement in mutants of Escherichia coli. Biochem Biophys Res Commun. 1973 Jan 23;50(2):551–558. doi: 10.1016/0006-291x(73)90875-9. [DOI] [PubMed] [Google Scholar]
- Wilson T. H., Kusch M. A mutant of Escherichia coli K 12 energy-uncoupled for lactose transport. Biochim Biophys Acta. 1972 Mar 17;255(3):786–797. doi: 10.1016/0005-2736(72)90391-4. [DOI] [PubMed] [Google Scholar]


