Abstract
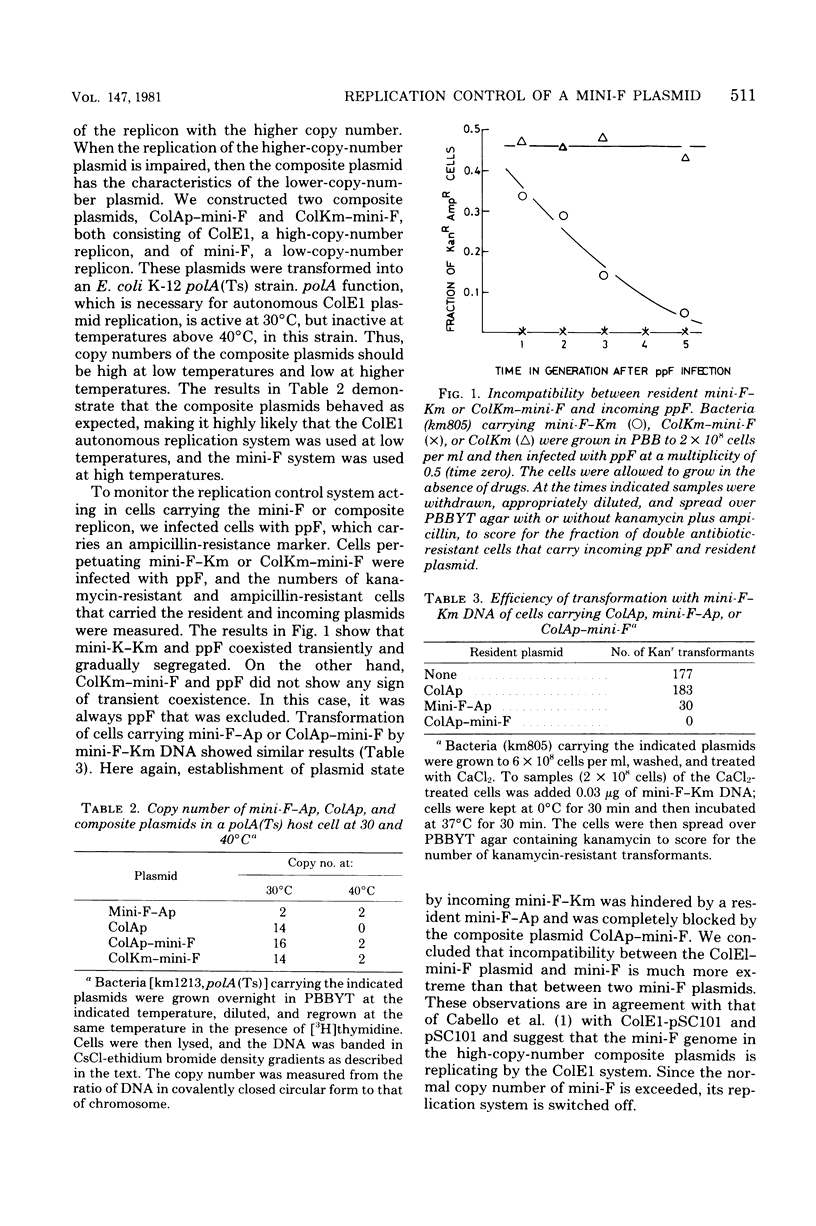
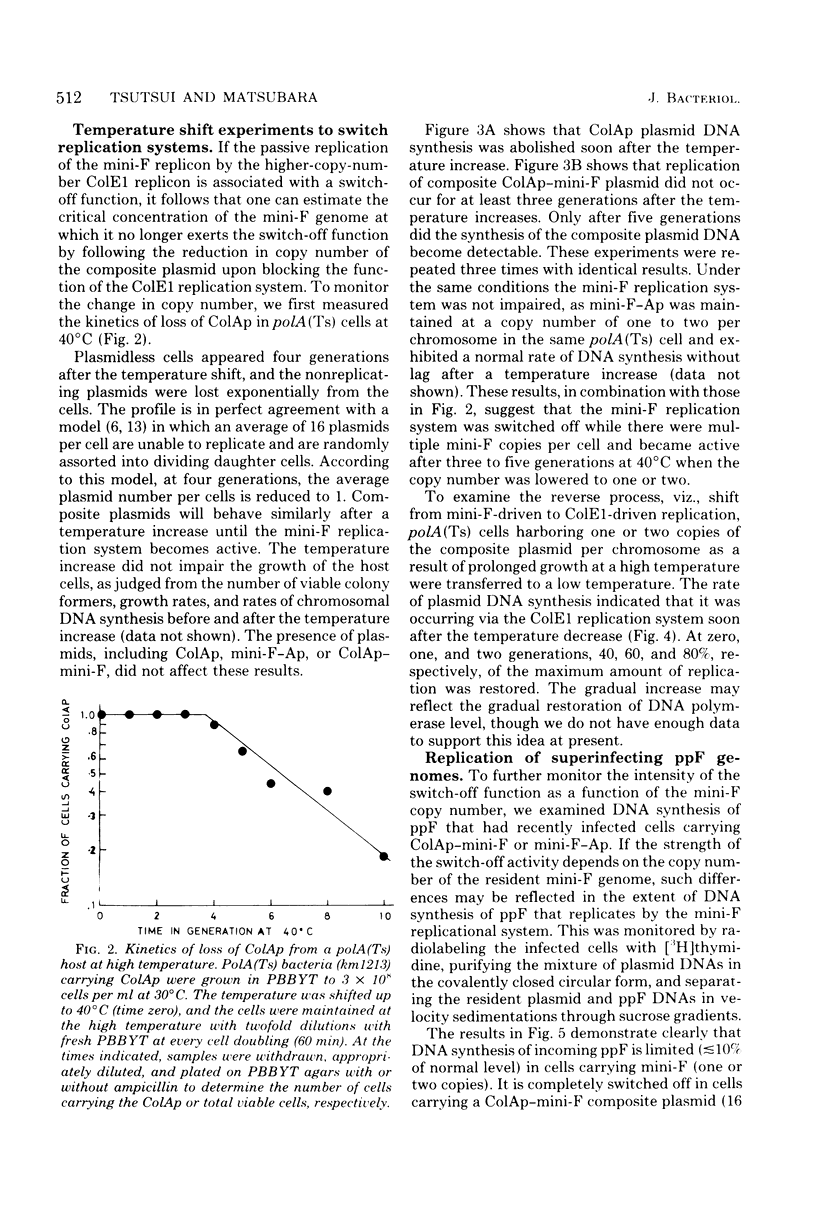
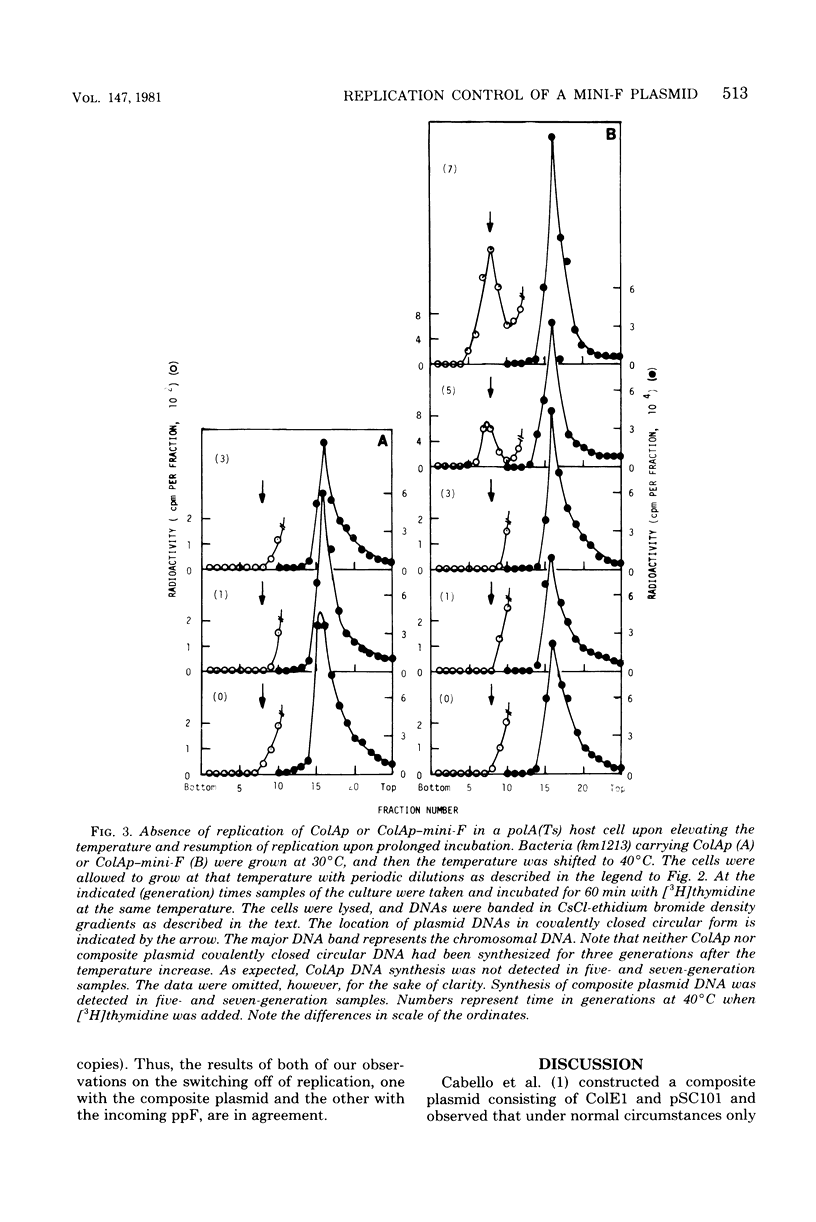
Mini-F is a fragment of the F plasmid, consisting of 9,000 base pairs, which carries all of the genes and sites required for replicon maintenance and control. Its copy number is one to two per chromosome. This plasmid is joined to ColE1, whose copy number is 16 to 20. Under normal circumstances the composite plasmid replication exhibited ColE1 characteristics, maintaining a high copy number. However, when ColE1 replication was inhibited by deoxyribonucleic acid polymerase I inactivation, its replication exhibited mini-F characteristics, maintaining a low copy number. These observations are in complete agreement with those of Timmis et al. (Proc. Natl. Acad. Sci. U.S.A. 71:4556-4560, 1974), who examined the behavior of a recombinant plasmid formed between pSC101 and ColE1. The transition from high to low copy number allowed us to examine the control system acting in cells carrying plasmids exhibiting intermediate copy numbers. The initiation of the mini-F replication system as represented by deoxyribonucleic acid synthesis of the composite plasmid was completely blocked when there were multiple copies of mini-F in a cell. It was not restored until the copy number was lowered to one to two, after which replication was first detected. ppF, a mini-F replicon packaged in a phage λ head behaved similarly: its replication was completely shut off when the resident mini-F genome copy number was high and was inhibited partially when the resident mini-F genome copy number was low. These experiments clearly demonstrate that there is a switch-off mechanism acting on deoxyribonucleic acid synthesis (initiation) in a cell carrying mini-F, and its intensity is related to the plasmid copy number. This result supports the “inhibitor dilution model” proposed by Pritchard et al. (Symp. Soc. Gen. Microbiol. 19:263-297, 1969). The nature of the hypothetical inhibitor is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabello F., Timmis K., Cohen S. N. Replication control in a composite plasmid constructed by in vitro linkage of two distinct replicons. Nature. 1976 Jan 29;259(5541):285–290. doi: 10.1038/259285a0. [DOI] [PubMed] [Google Scholar]
- Gustafsson P., Dreisig H., Molin S., Nordström K., Uhlin B. E. DNA replication control: studies of plasmid R1. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):419–425. doi: 10.1101/sqb.1979.043.01.048. [DOI] [PubMed] [Google Scholar]
- Gustafsson P., Nordström K. Temperature-dependent and amber copy mutants of plasmid R1drd-19 in Escherichia coli. Plasmid. 1978 Feb;1(2):134–144. doi: 10.1016/0147-619x(78)90034-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T., Inselburg J. ColE1 plasmid incompatibility: localization and analysis of mutations affecting incompatibility. J Bacteriol. 1979 Aug;139(2):608–619. doi: 10.1128/jb.139.2.608-619.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y., Matsubara K. Construction and some properties of packageable plasmid F. Mol Gen Genet. 1979 Jan 16;169(1):107–112. doi: 10.1007/BF00267551. [DOI] [PubMed] [Google Scholar]
- Ishii K., Hashimoto-Gotoh T., Matsubara K. Random replication and random assortment model for plasmid incompatibility in bacteria. Plasmid. 1978 Sep;1(4):435–445. doi: 10.1016/0147-619x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Method for the isolation of the replication region of a bacterial replicon: construction of a mini-F'kn plasmid. J Bacteriol. 1976 Aug;127(2):982–987. doi: 10.1128/jb.127.2.982-987.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K., Takagi Y., Mukai T. In vitro construction of different oligomeric forms of lambdadv DNA and studies of their transforming activities. J Virol. 1975 Sep;16(3):479–485. doi: 10.1128/jvi.16.3.479-485.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K., Takeda Y. Role of the tof gene in the production and perpetuation of the lambdadv plasmid. Mol Gen Genet. 1975 Dec 30;142(3):225–230. doi: 10.1007/BF00425647. [DOI] [PubMed] [Google Scholar]
- Molin S., Nordström K. Control of plasmid R1 replication: functions involved in replication, copy number control, incompatibility, and switch-off of replication. J Bacteriol. 1980 Jan;141(1):111–120. doi: 10.1128/jb.141.1.111-120.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Hoppensteadt F. C. On plasmid incompatibility. Plasmid. 1978 Sep;1(4):421–434. doi: 10.1016/0147-619x(78)90001-x. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Wabiko H., Tsurimoto T., Horii T., Masukata H., Ogawa H. Characteristics of purified recA protein and the regulation of its synthesis in vivo. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):909–915. doi: 10.1101/sqb.1979.043.01.099. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Hiraga S. F deoxyribonucleic acid superinfected into phenocopies of donor strains. J Bacteriol. 1975 Mar;121(3):1007–1013. doi: 10.1128/jb.121.3.1007-1013.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Andrés I., Slocombe P. M., Synenki R. M. Plasmid-encoded functions involved in the replication and inheritance of antibiotic-resistance plasmid R6-5. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):105–110. doi: 10.1101/sqb.1979.043.01.016. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Utilization of two distinct modes of replication by a hybrid plasmid constructed in vitro from separate replicons. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4556–4560. doi: 10.1073/pnas.71.11.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. A runaway-replication mutant of plasmid R1drd-19: temperature-dependent loss of copy number control. Mol Gen Genet. 1978 Oct 4;165(2):167–179. doi: 10.1007/BF00269904. [DOI] [PubMed] [Google Scholar]


