Abstract
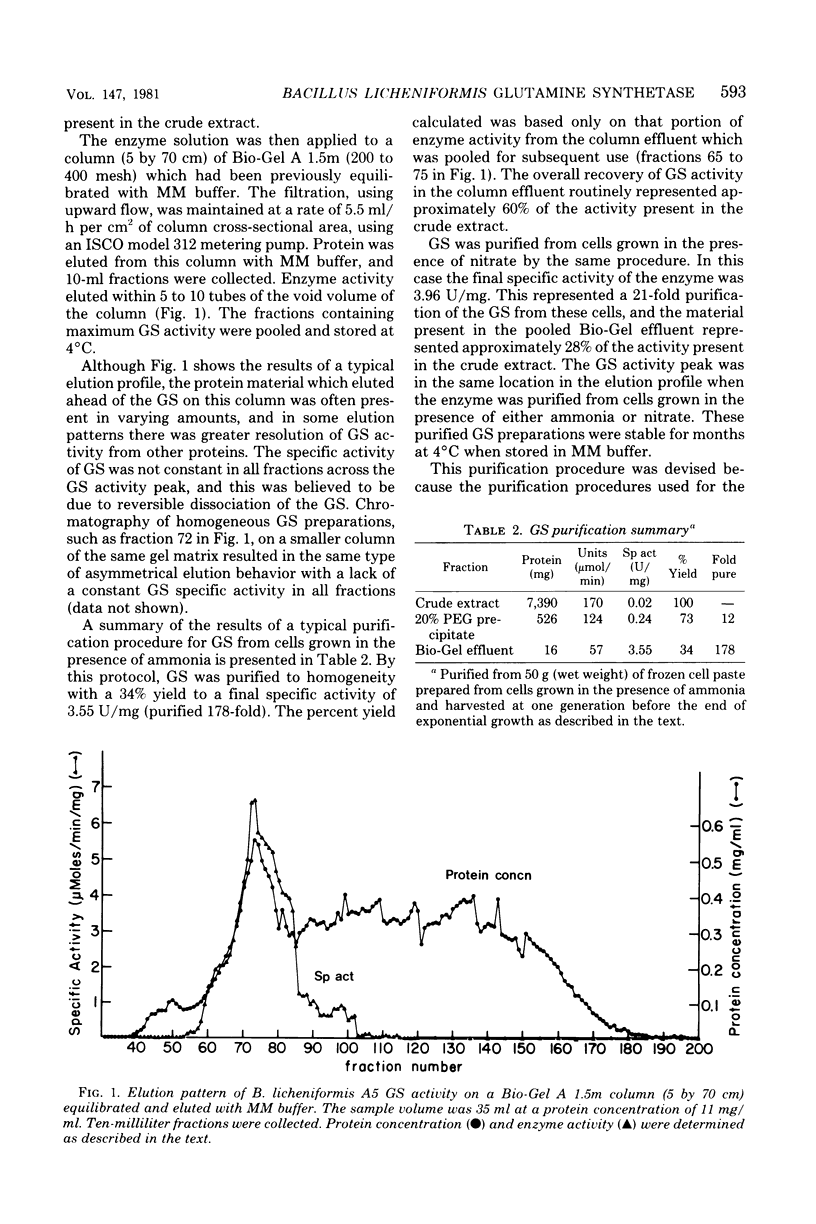
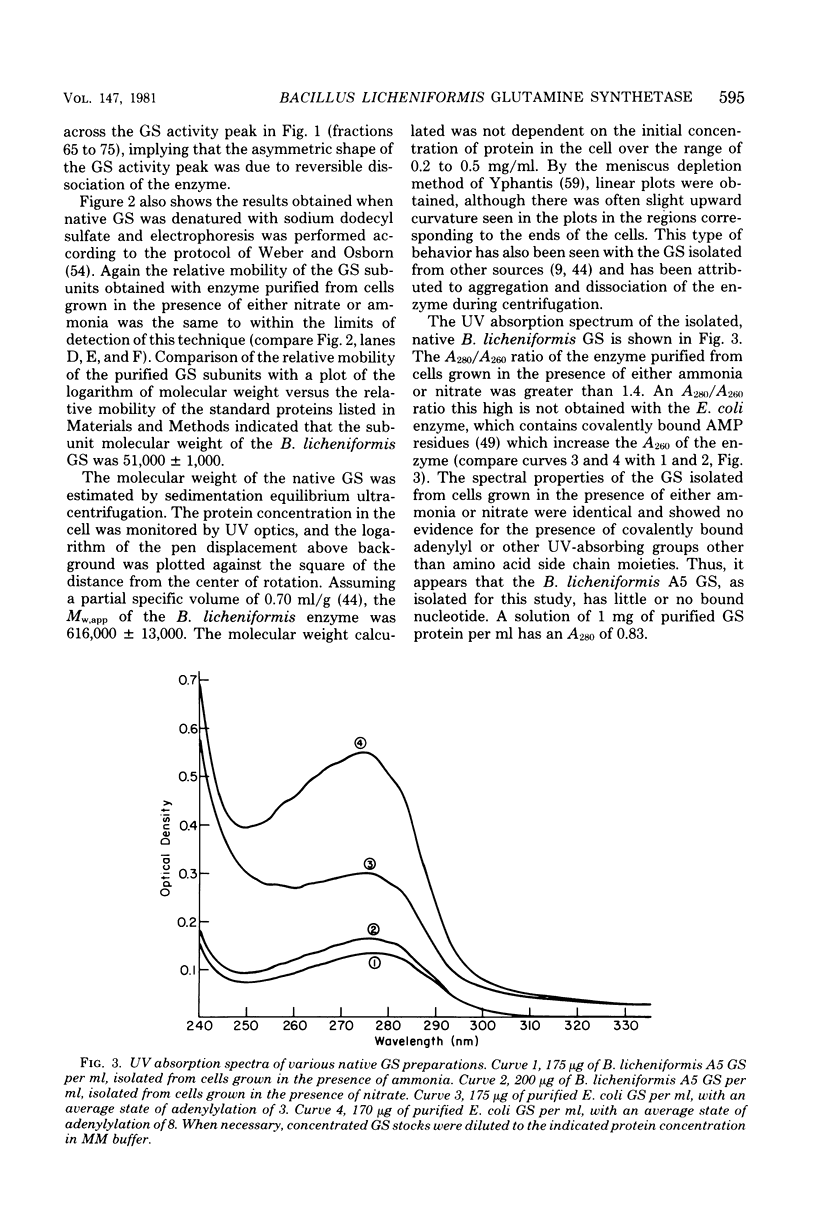
The glutamine synthetase from Bacillus licheniformis A5 was purified by using a combination of polyethylene glycol precipitation and chromatography on Bio-Gel A 1.5m. The resulting preparation was judged to be homogeneous by the criteria of polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate, equilibrium analytical ultracentrifugation, and electron microscopic analysis. The enzyme is a dodecamer with a molecular weight of approximately 616,000, and its subunit molecular weight is 51,000. Under optimal assay conditions (pH 6.6, 37 degrees C) apparent Km values for glutamate, ammonia, and manganese.adenosine 5'-triphosphate (1:1 ratio) were 3.6, 0.4, and 0.9 mM, respectively. Glutamine synthetase activity was inhibited approximately 50% by the addition of 5 mM glutamine, alanine, glycine, serine, alpha-ketoglutarate, carbamyl phosphate, adenosine 5'-diphosphate, or inosine 5'-triphosphate to the standard glutamine synthetase assay system, whereas 5 mM adenosine 5'-monophosphate or pyrophosphate caused approximately 90% inhibition of enzyme activity. Phosphorylribosyl pyrophosphate at 5 mM enhanced activity approximately 60%. We were unable to detect any physical or kinetic differences in the properties of the enzyme when it was purified from cells grown in the presence of ammonia or nitrate as sole nitrogen source. The data indicate that B. licheniformis A5 contains one species of glutamine synthetase whose catalytic activity is not regulated by a covalent modification system.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNLOHR R. W. POSTLOGARITHMIC PHASE METABOLISM OF SPORULATING MICROORGANISMS. I. PROTEASE OF BACILLUS LICHENIFORMIS. J Biol Chem. 1964 Feb;239:538–543. [PubMed] [Google Scholar]
- Baumberg S., Harwood C. R. Carbon and nitrogen repression of arginine catabolic enzymes in Bacillus subtilis. J Bacteriol. 1979 Jan;137(1):189–196. doi: 10.1128/jb.137.1.189-196.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott K. F., Reysset G., Gregoire J., Islert D., Aubert J. P. Characterization of glutamine requiring mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1977 Dec 7;79(3):996–1003. doi: 10.1016/0006-291x(77)91208-6. [DOI] [PubMed] [Google Scholar]
- Broman K., Lauwers N., Stalon V., Wiame J. M. Oxygen and nitrate in utilization by Bacillus licheniformis of the arginase and arginine deiminase routes of arginine catabolism and other factors affecting their syntheses. J Bacteriol. 1978 Sep;135(3):920–927. doi: 10.1128/jb.135.3.920-927.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow R. A., Knotts R. R. Two forms of glutamine synthetase in free-living root-nodule bacteria. Biochem Biophys Res Commun. 1977 Sep 23;78(2):554–559. doi: 10.1016/0006-291x(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Dawes I. W., Mandelstam J. Sporulation of Bacillus subtilis in continuous culture. J Bacteriol. 1970 Sep;103(3):529–535. doi: 10.1128/jb.103.3.529-535.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F. Bacillus subtilis glutamine synthetase. Specific catalytic changes associated with limited sulfhydryl modification. J Biol Chem. 1971 Feb 10;246(3):599–605. [PubMed] [Google Scholar]
- Deuel T. F., Ginsburg A., Yeh J., Shelton E., Stadtman E. R. Bacillus subtilis glutamine synthetase. Purification and physical characterization. J Biol Chem. 1970 Oct 25;245(20):5195–5205. [PubMed] [Google Scholar]
- Deuel T. F., Stadtman E. R. Some kinetic properties of Bacillus subtilis glutamine synthetase. J Biol Chem. 1970 Oct 25;245(20):5206–5213. [PubMed] [Google Scholar]
- Elmerich C., Aubert J. P. Role of glutamine synthetase in the repression of bacterial sporulation. Biochem Biophys Res Commun. 1972 Jan 31;46(2):892–897. doi: 10.1016/s0006-291x(72)80225-0. [DOI] [PubMed] [Google Scholar]
- Fisher S. H., Sonenshein A. L. Glutamine-requiring mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1977 Dec 7;79(3):987–995. doi: 10.1016/0006-291x(77)91207-4. [DOI] [PubMed] [Google Scholar]
- Foor F., Reuveny Z., Magasanik B. Regulation of the synthesis of glutamine synthetase by the PII protein in Klebsiella aerogenes. Proc Natl Acad Sci U S A. 1980 May;77(5):2636–2640. doi: 10.1073/pnas.77.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E. B., Vasantha N., Freese E. Induction of sporulation in developmental mutants of Bacillus subtilis. Mol Gen Genet. 1979 Feb 16;170(1):67–74. doi: 10.1007/BF00268581. [DOI] [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Fuchs R. L., Keister D. L. Identification of two glutamine synthetases in Agrobacterium. J Bacteriol. 1980 Feb;141(2):996–998. doi: 10.1128/jb.141.2.996-998.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn R., Oberrauch W., Mecke D. Activation of glutaminase by phosphoribosyl-pyrophosphate and its interference with the assay of phosphoribosylpyrophosphate amidotransferase. Biochim Biophys Acta. 1979 Jan 12;566(1):152–156. doi: 10.1016/0005-2744(79)90257-2. [DOI] [PubMed] [Google Scholar]
- Heinze J. E., Mitani T., Rich K. E., Freese E. Induction of sporulation by inhibitory purines and related compounds. Biochim Biophys Acta. 1978 Nov 21;521(1):16–26. doi: 10.1016/0005-2787(78)90245-9. [DOI] [PubMed] [Google Scholar]
- Hubbard J. S., Stadtman E. R. Regulation of glutamine synthetase. V. Partial purification and properties of glutamine synthetase from Bacillus licheniformis. J Bacteriol. 1967 Oct;94(4):1007–1015. doi: 10.1128/jb.94.4.1007-1015.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. S., Stadtman E. R. Regulation of glutamine synthetase. VI. Interactions of inhibitors for Bacillus licheniformis glutamine synthetase. J Bacteriol. 1967 Oct;94(4):1016–1024. doi: 10.1128/jb.94.4.1016-1024.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Kane J. F., Deshpande K. L. Properties of glutamate dehydrogenase from Bacillus subtilis. Biochem Biophys Res Commun. 1979 Jun 13;88(3):761–767. doi: 10.1016/0006-291x(79)91473-6. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Kleiner D. The glutamine synthetase from Azotobacter vinelandii: purification, characterization, regulation and localization. Eur J Biochem. 1978 Aug 15;89(1):51–60. doi: 10.1111/j.1432-1033.1978.tb20895.x. [DOI] [PubMed] [Google Scholar]
- LEONARD C. G., HOUSEWRIGHT R. D., THORNE C. B. Effects of metal ions on the optical specificity of glutamine synthetase and glutamyl transferase of Bacillus licheniformis. Biochim Biophys Acta. 1962 Aug 13;62:432–434. doi: 10.1016/0006-3002(62)90278-0. [DOI] [PubMed] [Google Scholar]
- Laishley E. J., Bernlohr R. W. Catabolite repression of "three sporulation enzymes" during growth of Bacillus licheniformis. Biochem Biophys Res Commun. 1966 Jul 6;24(1):85–90. doi: 10.1016/0006-291x(66)90414-1. [DOI] [PubMed] [Google Scholar]
- Ludwig R. A. Physiological roles of glutamine synthetases I and II in ammonium assimilation in Rhizobium sp. 32H1. J Bacteriol. 1980 Mar;141(3):1209–1216. doi: 10.1128/jb.141.3.1209-1216.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Munoz L., Doi R. H. A procedure to remove protease activities from Bacillus subtilis sporulating cells and their crude extracts. Anal Biochem. 1977 Mar;78(1):165–170. doi: 10.1016/0003-2697(77)90020-3. [DOI] [PubMed] [Google Scholar]
- Orr M. D., Blakley R. L., Panagou D. Discontinuous buffer systems for analytical and preparative electrophoresis of enzymes on polyacrylamide gel. Anal Biochem. 1972 Jan;45(1):68–85. doi: 10.1016/0003-2697(72)90008-5. [DOI] [PubMed] [Google Scholar]
- Pahel G., Tyler B. A new glnA-linked regulatory gene for glutamine synthetase in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4544–4548. doi: 10.1073/pnas.76.9.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F. L., Coote J. G. Glutamine synthetase and glutamate synthase activities during growth and sporulation in Bacillus subtilis. J Gen Microbiol. 1979 Jun;112(2):373–377. doi: 10.1099/00221287-112-2-373. [DOI] [PubMed] [Google Scholar]
- Paulus T. J., Switzer R. L. Characterization of pyrimidine-repressible and arginine-repressible carbamyl phosphate synthetases from Bacillus subtilis. J Bacteriol. 1979 Jan;137(1):82–91. doi: 10.1128/jb.137.1.82-91.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phibbs P. V., Jr, Bernlohr R. W. Purification, properties, and regulation of glutamic dehydrogenase of Bacillus licheniformis. J Bacteriol. 1971 May;106(2):375–385. doi: 10.1128/jb.106.2.375-385.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pishak M. R., Phillips A. T. A modified radioisotopic assay for measuring glutamine synthetase activity in tissue extracts. Anal Biochem. 1979 Apr 1;94(1):82–88. doi: 10.1016/0003-2697(79)90793-0. [DOI] [PubMed] [Google Scholar]
- Prusiner S., Milner L. A rapid radioactive assay for glutamine synthetase, glutaminase, asparagine synthetase, and asparaginase. Anal Biochem. 1970 Oct;37(2):429–438. doi: 10.1016/0003-2697(70)90069-2. [DOI] [PubMed] [Google Scholar]
- Rebello J. L., Strauss N. Regulation of synthesis of glutamine synthase in Bacillus subtilis. J Bacteriol. 1969 May;98(2):683–688. doi: 10.1128/jb.98.2.683-688.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reysset G., Aubert J. P. Relationship between sporulation and mutations impairing glutamine synthetase in Bacillus megaterium. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1237–1241. doi: 10.1016/s0006-291x(75)80362-7. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Ginsburg A. Effects of specific divalent cations on some physical and chemical properties of glutamine synthetase from Escherichia coli. Taut and relaxed enzyme forms. Biochemistry. 1968 Jun;7(6):2153–2167. doi: 10.1021/bi00846a018. [DOI] [PubMed] [Google Scholar]
- Siedel J., Shelton E. Purification and properties of Azotobacter vinelandii glutamine synthetase. Arch Biochem Biophys. 1979 Jan;192(1):214–224. doi: 10.1016/0003-9861(79)90086-9. [DOI] [PubMed] [Google Scholar]
- Siegel W. H., Donohue T., Bernlohr R. W. Determination of pools of tricarboxylic acid cycle and related acids in bacteria. Appl Environ Microbiol. 1977 Nov;34(5):512–517. doi: 10.1128/aem.34.5.512-517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey G., Van Baalen C., Tabita F. R. Nitrogen and ammonia assimilation in the cyanobacteria: regulation of glutamine synthetase. Arch Biochem Biophys. 1979 May;194(2):457–467. doi: 10.1016/0003-9861(79)90640-4. [DOI] [PubMed] [Google Scholar]
- Stasiewicz S., Dunham V. L. Isolation and characterization of two forms of glutamine synthetsae from soybean hypocotyl. Biochem Biophys Res Commun. 1979 Mar 30;87(2):627–634. doi: 10.1016/0006-291x(79)91840-0. [DOI] [PubMed] [Google Scholar]
- Streicher S. L., Tyler B. Purification of glutamine synthetase from a variety of bacteria. J Bacteriol. 1980 Apr;142(1):69–78. doi: 10.1128/jb.142.1.69-78.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen F. W., Bernlohr R. W. Pyruvate kinase of the spore-forming bacterium, Bacillus licheniformis. I. Purification, stability, regulation of synthesis, and evidence for multiple molecular states. J Biol Chem. 1971 Mar 25;246(6):1733–1745. [PubMed] [Google Scholar]
- Tyler B. Regulation of the assimilation of nitrogen compounds. Annu Rev Biochem. 1978;47:1127–1162. doi: 10.1146/annurev.bi.47.070178.005403. [DOI] [PubMed] [Google Scholar]
- Wedler F. C., Hoffmann F. M. Glutamine synthetase of Bacillus stearothermophilus. I. Purification and basic properties. Biochemistry. 1974 Jul 30;13(16):3207–3214. doi: 10.1021/bi00713a002. [DOI] [PubMed] [Google Scholar]
- Wedler F. C., Kenney R. M., Ashour A. E., Carfi J. Two regulatory isozymes of glutamine synthetase from Bacillus caldolyticus, an extreme thermophile. Biochem Biophys Res Commun. 1978 Mar 15;81(1):122–126. doi: 10.1016/0006-291x(78)91638-8. [DOI] [PubMed] [Google Scholar]
- Wedler F. C., Shreve D. S., Kenney R. M., Ashour A. E., Carfi J., Rhee S. G. Two glutamine synthetases from Bacillus caldolyticus, an extreme thermophile. Isolation, physicochemical and kinetic properties. J Biol Chem. 1980 Oct 10;255(19):9507–9516. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]




