Abstract
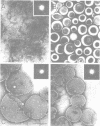
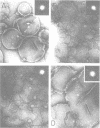
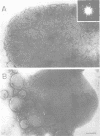
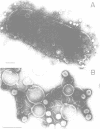
The LamB protein purified in a solution of sodium dodecyl sulfate was assembled into an ordered hexagonal lattice structure with a lattice constant of about 7.8 nm in the presence of lipopolysaccharide. The LamB alone formed aggregates with some lattice structure. However, the regularity of the lattice was only maintained within a very small area. An ordered hexagonal lattice was also formed when the wild-type lipopolysaccharide was replaced by heptoseless lipopolysaccharide, lipid A, and even fatty acid. However, the lattice constants were appreciably smaller than that with the wild-type lipopolysaccharide. The results suggest that the heptose-containing polysaccharide region, as well as the fatty acid region, are involved in the interaction with the LamB protein. The LamB-lipopolysaccharide lattice was preferably formed on the peptidoglycan layer when the lipoprotein was covalently bound to this layer. These results indicate that the molecular arrangement of the LamB protein in the outer membrane is similar to that of matrix proteins, OmpC and OmpF, which exist as trimers. The ordered hexagonal lattice was active in the receptor function for lambda, resulting in phage adsorption and deoxyribonucleic acid ejection. Thus, this reconstitution system should provide a useful means of studying the mechanism of lambda infection.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Boehler-Kohler B. A., Boos W., Dieterle R., Benz R. Receptor for bacteriophage lambda of Escherichia coli forms larger pores in black lipid membranes than the matrix protein (porin). J Bacteriol. 1979 Apr;138(1):33–39. doi: 10.1128/jb.138.1.33-39.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch V., Braun V. Distribution of murein-lipoprotein between the cytoplasmic and outer membrane of Escherichia coli. FEBS Lett. 1973 Aug 15;34(2):307–310. doi: 10.1016/0014-5793(73)80818-x. [DOI] [PubMed] [Google Scholar]
- Braun V., Krieger-Brauer H. J. Interrelationship of the phage lambda receptor protein and maltose transport in mutants of Escherichia coli K12. Biochim Biophys Acta. 1977 Aug 15;469(1):89–98. doi: 10.1016/0005-2736(77)90328-5. [DOI] [PubMed] [Google Scholar]
- DeMartini M., Inouye M. Interaction between two major outer membrane proteins of Escherichia coli: the matrix protein and the lipoprotein. J Bacteriol. 1978 Jan;133(1):329–335. doi: 10.1128/jb.133.1.329-335.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edermann R., Hindennach I., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane. Preliminary characterization of the phage lambda receptor protein. FEBS Lett. 1978 Apr 1;88(1):71–74. doi: 10.1016/0014-5793(78)80609-7. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Voter W. A., Leonard K. Image reconstruction in electron microscopy: enhancement of periodic structure by optical filtering. Methods Enzymol. 1978;49:39–63. doi: 10.1016/s0076-6879(78)49006-8. [DOI] [PubMed] [Google Scholar]
- Furukawa H., Yamada H., Mizushima S. Interaction of bacteriophage T4 with reconstituted cell envelopes of Escherichia coli K-12. J Bacteriol. 1979 Dec;140(3):1071–1080. doi: 10.1128/jb.140.3.1071-1080.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O. Interaction of lipopolysaccharides and lipid A with complement. Eur J Biochem. 1971 Mar 1;19(1):143–152. doi: 10.1111/j.1432-1033.1971.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y., Yamada H., Mizushima S. Interactions of outer membrane proteins O-8 and O-9 with peptidoglycan sacculus of Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1401–1409. doi: 10.1093/oxfordjournals.jbchem.a131413. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara S., Mizushima S. Arrangement of proteins O-8 and O-9 in outer membrane of Escherichia coli K-12. Existence of homotrimers and heterotrimers. Eur J Biochem. 1979 Oct 15;100(2):321–328. doi: 10.1111/j.1432-1033.1979.tb04174.x. [DOI] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl P. W. The receptor of bacteriophage lambda: evidence for its dimeric nature. FEBS Lett. 1978 Dec 15;96(2):385–388. doi: 10.1016/0014-5793(78)80443-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee N., Inouye M. Outer membrane proteins of Escherichia coli: biosynthesis and assembly. FEBS Lett. 1974 Feb 15;39(2):167–170. doi: 10.1016/0014-5793(74)80043-8. [DOI] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Peters R., Bernheimer H., Berendsen W. Influence of cultural conditions and mutations on the composition of the outer membrane proteins of Escherichia coli. Mol Gen Genet. 1976 Sep 23;147(3):251–262. doi: 10.1007/BF00582876. [DOI] [PubMed] [Google Scholar]
- Mackay D. J., Bode V. C. Binding to isolated phage receptors and lambda DNA release in vitro. Virology. 1976 Jul 1;72(1):167–181. doi: 10.1016/0042-6822(76)90321-4. [DOI] [PubMed] [Google Scholar]
- Moreno F., Wandersman C. OmpC and LamB proteins can serve as substitute receptors for host range mutants of coliphage TuIa. J Bacteriol. 1980 Dec;144(3):1182–1185. doi: 10.1128/jb.144.3.1182-1185.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh N., Furukawa H., Mizushima S. Role of lipopolysaccharide and outer membrane protein of Escherichia coli K-12 in the receptor activity for bacteriophage T4. J Bacteriol. 1978 Nov;136(2):693–699. doi: 10.1128/jb.136.2.693-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. A porin activity of purified lambda-receptor protein from Escherichia coli in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1979 Jun 13;88(3):774–781. doi: 10.1016/0006-291x(79)91475-x. [DOI] [PubMed] [Google Scholar]
- Nakae T., Ishii J., Tokunaga M. Subunit structure of functional porin oligomers that form permeability channels in the other membrane of Escherichia coli. J Biol Chem. 1979 Mar 10;254(5):1457–1461. [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. In vitro reassembly of the membranous vesicle from Escherichia coli outer membrane components. Role of individual components and magnesium ions in reassembly. Biochim Biophys Acta. 1975 Dec 16;413(3):371–393. doi: 10.1016/0005-2736(75)90122-4. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Randall L. L. Arrangement of protein I in Escherichia coli outer membrane: cross-linking study. J Bacteriol. 1978 Jan;133(1):279–286. doi: 10.1128/jb.133.1.279-286.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Westermann P. Arrangement of the maltose-inducible major outer membrane proteins, the bacteriophage lambda receptor in Escherichia coli and the 44 K protein in Salmonella typhimurium. FEBS Lett. 1979 Mar 1;99(1):77–80. doi: 10.1016/0014-5793(79)80253-7. [DOI] [PubMed] [Google Scholar]
- Prehm P., Stirm S., Jann B., Jann K., Boman H. G. Cell-wall lipopolysaccharides of ampicillin-resistant mutants of Escherichia coli K-12. Eur J Biochem. 1976 Jul 1;66(2):369–377. doi: 10.1111/j.1432-1033.1976.tb10526.x. [DOI] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L. Quantitation of the loss of the bacteriophage lambda receptor protein from the outer membrane of lipopolysaccharide-deficient strains of Escherichia coli. J Bacteriol. 1975 Jul;123(1):41–46. doi: 10.1128/jb.123.1.41-46.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Szmelcman S., Hofnung M. Maltose transport in Escherichia coli K-12: involvement of the bacteriophage lambda receptor. J Bacteriol. 1975 Oct;124(1):112–118. doi: 10.1128/jb.124.1.112-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Schwartz M., Silhavy T. J., Boos W. Maltose transport in Escherichia coli K12. A comparison of transport kinetics in wild-type and lambda-resistant mutants as measured by fluorescence quenching. Eur J Biochem. 1976 May 17;65(1):13–19. doi: 10.1111/j.1432-1033.1976.tb10383.x. [DOI] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Interaction between major outer membrane protein (O-8) and lipopolysaccharide in Escherichia coli K12. Eur J Biochem. 1980 Jan;103(1):209–218. doi: 10.1111/j.1432-1033.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Lipoprotein-bearing peptidoglycan sacculus as a preferred site for the in vitro assembly of membrane from dissociated components of outer membrane of Escherichia coli K-12. J Biochem. 1977 Jun;81(6):1889–1899. doi: 10.1093/oxfordjournals.jbchem.a131651. [DOI] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Reconstitution of an ordered structure from major outer membrane constituents and the lipoprotein-bearing peptidoglycan sacculus of Escherichia coli. J Bacteriol. 1978 Sep;135(3):1024–1031. doi: 10.1128/jb.135.3.1024-1031.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Ichihara S., Mizushima S. A major outer membrane protein (O-8) of Escherichia coli K-12 exists as a trimer in sodium dodecyl sulfate solution. FEBS Lett. 1979 Apr 1;100(1):71–74. doi: 10.1016/0014-5793(79)81133-3. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Nikaido H. Outer membrane of gram-negative bacteria. XVII. Secificity of transport process catalyzed by the lambda-receptor protein in Escherichia coli. Biochem Biophys Res Commun. 1977 Oct 10;78(3):1100–1107. doi: 10.1016/0006-291x(77)90534-4. [DOI] [PubMed] [Google Scholar]