Abstract
The polyamines are small organic cations that are absolutely required for eukaryotic cell growth. Although their growth requirements are well established, the molecular functions of the polyamines are ill-defined. Oxidative damage to DNA by reactive oxygen species is a continual problem that cells must guard against to survive. The polyamine spermine, which is normally found in millimolar concentrations in the nucleus, is shown here to function directly as a free radical scavenger, and adducts formed as a result of this function are identified. These data suggest that spermine is a major natural intracellular compound capable of protecting DNA from free radical attack.
The cellular polycationic polyamines are ubiquitous in nature and are absolutely required for eukaryotic cell growth (1, 2). However, very few specific molecular functions of polyamines have been described (3, 4). Some of the attributes ascribed to polyamines, particularly spermine, include the regulation of gene expression (5), the stabilization of chromatin (6–8), the prevention of endonuclease-mediated DNA fragmentation (9), and the inhibition of DNA damage (8, 10–13). Lovaas has recently reviewed many of these properties (14). Although protection of DNA damage by polyamines has been demonstrated, the mechanisms by which spermine functions in this protection are not known. Most of the proposals suggest that polyamine action is a result of charge neutralization and conformational changes (8, 15). Because spermine is thought to be intimately associated with chromatin (7), we sought to investigate if spermine had a direct role in protecting DNA from free radical attack. Free radical and other reactive oxygen species (ROS) generation through normal cellular metabolism and by exogenous insult is a constant problem for which cells have developed multiple protective mechanisms to survive(16). Here we demonstrate that spermine, which is normally found in millimolar concentrations in the nucleus (17), can function directly as a free radical scavenger.
MATERIALS AND METHODS
Assays for DNA Strand Breaks.
DNA strand breakage was measured by the conversion to open circular and linear forms of supercoiled ΦX-174 RF1 double-stranded DNA (New England Biolabs). To assess DNA cleavage, 0.2 μg of DNA was incubated in the presence of 30 μM H2O2 and 10 μM CuCl2 in PBS (pH 7.4) in a total volume of 30 μl as described (18, 19). Polyamines or antioxidants were coincubated as indicated. Following incubation the samples were separated by electrophoresis in a 1% agarose gel containing 40 mM Tris-acetate and 1 mM EDTA in a horizontal slab gel apparatus using Tris/acetate gel buffer. The gel was stained with ethidium bromide (2 μg/ml) for 10 min, followed by destaining in water for 10 min, and was then photographed by UV translumination. The gels were photographed using an Eagle Eye digital camera (Stratagene). A single strand break in supercoiled double-stranded DNA results in the formation of open circular DNA, and double strand breaks result in the formation of linear DNA. In agarose gel electrophoresis of untreated ΦX-174 plasmid DNA, supercoiled DNA migrates faster than open circular DNA. The open circular form migrates more slowly than the linear form due to the loss of compactness by superhelicity.
Electron Paramagnetic Resonance (EPR) Analysis.
5,5-Dimethyl-1-pyrroline-N-oxide (DMPO, 97%; Aldrich) was further purified by distillation. For the standard assay, 30 μM H2O2 and 10 μM Cu(II), in PBS (pH 7.4), and 50 mM DMPO were combined with or without the indicated concentration of spermine. Spectra were recorded after a 1-hr incubation time. Spectra were recorded on a Bruker ER 300 spectrometer operating in the X-band with a TM cavity as previously reported in ref. 20. The spectrometer settings used were: modulation frequency, 100 kHz; modulation amplitude, 0.5 G; scan time, 10 min.; microwave power, 20 mW; microwave frequency, 9.782 GHz.
Synthesis and Analysis of Bis-α-[13C]spermine for NMR and MS Analysis.
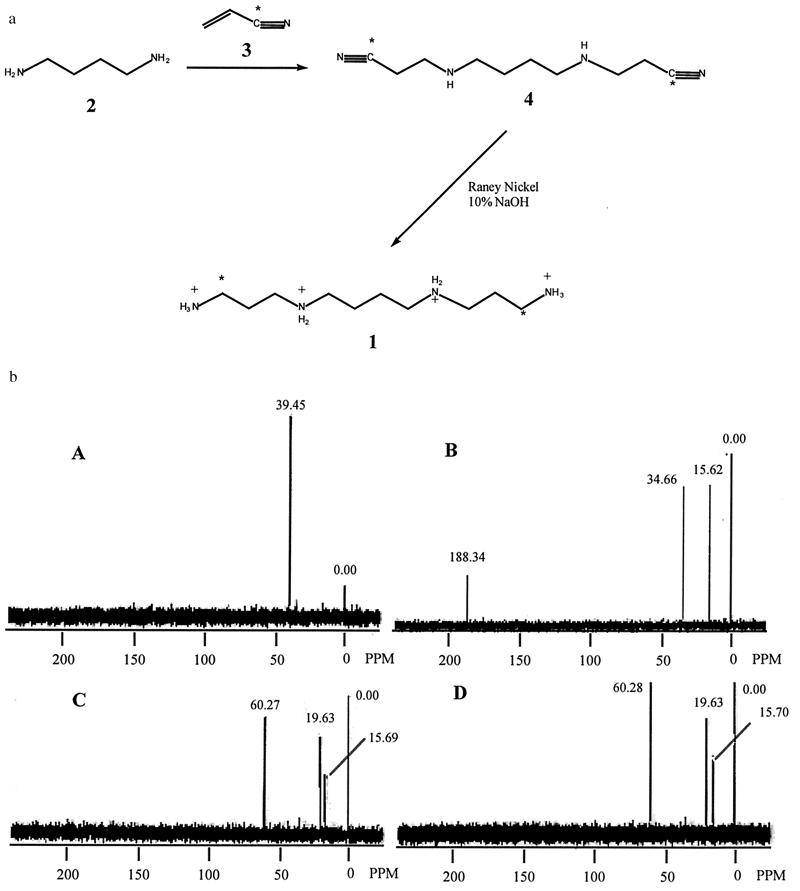
Bis-α-[13C]spermine synthesis (Fig. 3a) was initiated by the bis-cyanoethylation of 1,4-diaminobutane (2) with 9%-[13C]CN-enriched acrylonitrile (3) in refluxing ethanol (21). This reaction produced the corresponding 1,10-dicyano-3,8-diazadecane (4), which was reduced with Raney nickel (22), producing crude bis-α-[13C]spermine. The labeled spermine was purified by crystallization of the tetrahydrochloride salt from ethanol/water (21, 23).
Figure 3.
(a). Synthetic scheme for bis-α-[13C]spermine. 1,10-Bis([13C]Cyano)-3,8-diazadecane (4). A 0.198-g portion of 1,4-diaminobutane (2) (0.00224 mol) was added to a solution of 0.250 g (0.00465 mol, 2.07-fold excess) of [13C]cyano-enriched acrylonitrile (3) in 6 ml of ethanol, and the reaction was allowed to reflux for 18 hr. The solvent was then removed under reduced pressure, and the dark brown, oily residue was purified on silica gel (4 × 8 cm column, CHCl3:MeOH:NH4OH 900:600:3) to afford 0.261 g of a pale yellow oil, with an Rf of 0.41 (59.3% yield). 1H-NMR (CDCl3, ppm δ 1.54 (t, 4h, H5 and H6), 2.10 (s, 1H, NH), 2.56 (q, 4H, H4 and H7), 2.65 (t, 4H, H2 and H9), 2.91 (t, 4H, H1 and H10); IR cm−1 3306 (NH), 2938 (aliphatic), 2198 (CN); 13C-NMR CDCl3, ppm δ 118.6 (CN). Bis(δ-13C)-spermine (1). A 0.261-g portion of (4) and 0.267 g of NaOH were added to a suspension of 0.458 g of Raney nickel in 20 ml of dry ethanol, and the mixture was hydrogenated (Parr apparatus) at 50 psi for 24 hr. The reaction was then filtered (Zetapore 0.45 δ) and the solvent was removed under reduced pressure. The yellow solid residue was dissolved in 30 ml of water and washed with three 25-ml portions of chloroform. The aqueous layer was then acidified to pH 1, and the solution was concentrated under reduced pressure to afford 0.56 g of a white solid. Recrystallization of this solid from water/ethanol afforded 0.41 g (89.1%) of bis-(α-13C)-spermine (1) as the tetrachloride salt. 1H-NMR (D2O, ppm) δ = 1.77 (t, 4h, H6 and H7), 2.08 (m, 4H, H2 and H11), 3.11 (t, 12H, H1, H3, H5, H8, H10 and H12); 13C-NMR (D2O, ppm) δ = 36.5 (13CH2—NH3). (b) NMR data for [13C]spermine free radical scavenging experiments. Spectrum A: bis-α-13C-enriched spermine. Spectrum B: 1% TSP in D2O. Spectrum C: 1 mM bis-α-13C-enriched spermine plus 0.1 M H2O2, 33.3 mM CuCl2. Spectrum D: 0.5 mM bis-α-13C-enriched spermine plus 0.5 M H2O2, 167 mM CuCl2. All spectra were recorded on a General Electric QE 300 mHz FT-NMR, in D2O.
RESULTS AND DISCUSSION
Polyamines have been proposed to protect DNA from ROS damage. However, the mechanisms by which this occurs are unknown. Khan et al. (10, 11) proposed a mechanism by which spermidine and spermine could protect DNA from singlet oxygen attack, but did not demonstrate a direct involvement of the polyamines in the protection. The very reactive hydroxyl radicals can be generated by the reaction of hydrogen peroxide with DNA-associated transition metals, such as copper, in a Fenton-type mechanism: H2O2 + Cu(I) → OH⋅ + OH− + Cu(II). Hydroxyl radical formation mediated by transition metals is hypothesized to be an important physiological condition, and is thought to be responsible for oxidative toxicity in vivo, rather than H2O2 itself. To determine if spermine is capable of protecting DNA against ROS-induced strand breakage, we used φX-174 plasmid DNA and a Cu(II)/H2O2-dependent oxygen-radical generating system (24).
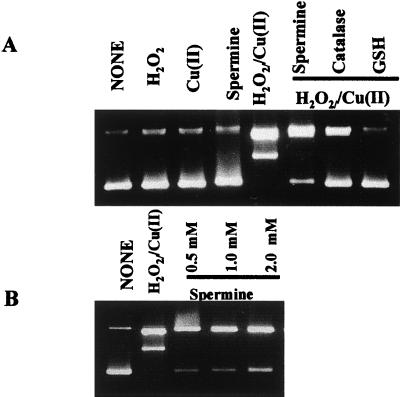
When ΦX-174 plasmid DNA was incubated in the presence of the ROS-generating system, no supercoiled DNA was observed to remain, indicating ROS attack of DNA leading to strand breaks and release of supercoiled DNA to open circle and the production of linear DNA. Neither Cu(II) nor H2O2 alone induced DNA strand breaks. Spermine at physiologically relevant concentrations inhibited ROS-induced DNA damage, with maximal inhibition observed at 1 to 2 mM (Fig. 1). These concentrations are well within the estimates for the physiological concentrations of spermine (17, 25). These results suggest that spermine protects DNA from ROS attack in a manner similar to the classical antioxidants.
Figure 1.
Protection of ROS-induced DNA damage by spermine. ΦX-174 plasmid DNA (200 ng) was incubated in the ROS-generating system. (A) Where indicated, spermine, 1 mM catalase (500 units/ml) or reduced glutathione (4 mM). (B) Protective effects of increasing concentrations of spermine. O, open circle; L, Linear; SC, supercoiled DNA.
There are at least three possible mechanisms by which spermine could reduce oxidative damage in this system. First, the association of spermine with DNA could lead to a conformational change in DNA, making it less susceptible to attack. Second, in some instances, spermine could chelate the transition metal, thus reducing the amount of ROS produced. However, this is not likely at physiological pH. Third, spermine can act directly as a free radical scavenger.
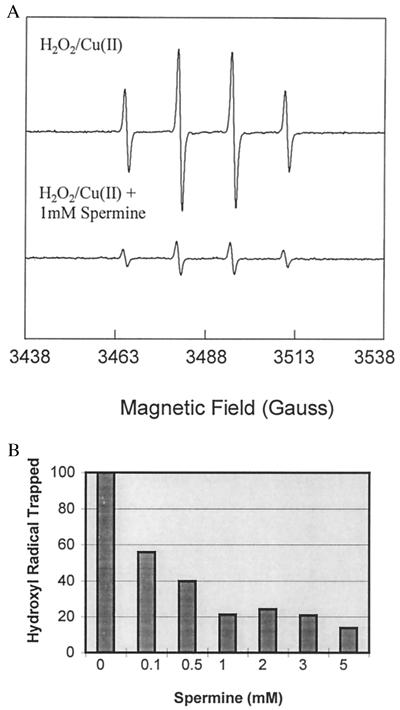
To eliminate the first possibility, that the protective effects of spermine were solely based on spermine-induced DNA conformational changes, DNA was removed from the system. The effects of spermine addition to the ROS-generating system were measured by EPR spectroscopy using DMPO as the spin trap (19). EPR analysis of the Cu(II)/H2O2-dependent oxygen-radical generating system in the absence of DNA displayed a 1:2:2:1 quartet signal indicative of the DMPO—OH spin adduct formation. The addition of 1 mM spermine effectively inhibited the Cu(II)/H2O2-mediated generation of the DMPO—OH adducts (>70%), supporting the role of spermine as an antioxidant (Fig. 2a). Spermine reduced the DMPO—OH adduct at concentrations as low as 0.1 mM (Fig. 2B). The DMPO—OH adduct formation was completely inhibited by the coaddition of the Cu(I) chelator bathocuproinedisulfonic acid, and was significantly diminished by the coaddition of catalase, directly indicating that the reaction was dependent on copper redox activity and H2O2 in the phosphate-buffered system (not shown). It should be noted that Hanna et al. (26) have demonstrated that DMPO—OH adducts can be formed by the nucleophilic addition of water to DMPO in the presence of copper, and suggest that this nucleophilic addition could be up to 80% of the total DMPO—OH adduct signal produced in the Cu/H2O2-dependent oxygen-radical generating system (27). However, copper alone did not induce DNA strand breaks, and spermine was also shown to reduce the DMPO—OH adduct formation produced in Fe-nitrilotriacetic acid (Fe-NTA)/H2O2, where no nucleophilic addition occurs (26) (not shown). These results suggest that spermine reduces the DMPO—OH adduct formation resulting from the spin trapping of hydroxyl radicals, and that the reduction of DMPO—OH adduct formation does not occur simply by chelating transition metals.
Figure 2.
Effects of spermine on the hydroxyl radical spin-trapping by DMPO in the ROS-generating system. (A) Upper EPR spectrum is from a sample containing 30 μM H2O2 + 10 μM Cu(II). Lower EPR spectrum is from a sample containing 30 μM H2O2 + 10 μM Cu(II) + 1 mM spermine. Incubation of H2O2 without Cu(II) or the addition of bathocuproinedisulfonic acid, a Cu(I) chelator, to the H2O2/Cu(II) mixture completely inhibited the formation of the DMPO—OH quartet (not shown). These results are representative of at least three sets of experiments. (B) Effects of increasing concentrations of spermine on DMPO—OH formation in the ROS-generating system.
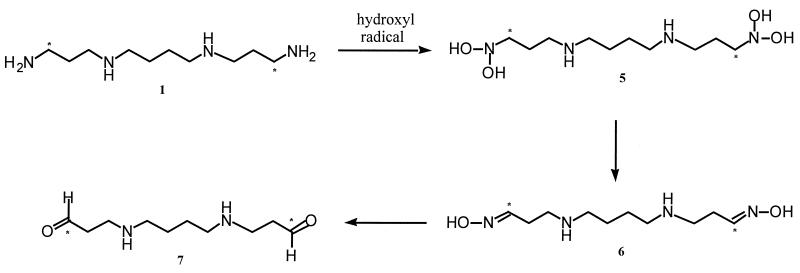
Although the above results are consistent with the hypothesis that spermine is capable of reducing hydroxyl radical attack without reliance on changes in DNA conformation, suggesting that hydroxyl radical and other ROS could react directly with spermine, they are not conclusive. Specifically, they do not exclude entirely the possibility that the EPR data are the result of copper chelation by spermine. Therefore, nuclear magnetic resonance (NMR) spectroscopy was used to determine if adducts of spermine were formed in the ROS-generating system, and as a preliminary step toward identifying those adducts. For use as a probe in NMR studies, bis-α-[13C]spermine was synthesized (Fig. 3a). The resulting 13C-enriched spermine was subjected to the same oxygen-radical generating system described above in the absence of DNA. The products of this reaction were then analyzed by 13C-NMR, using sodium 3-(trimethylsilyl)propionate (TSP) as internal standard. The enriched spermine spectrum showed a single resonance (total of 10 acquisitions) at 39 ppm (Fig. 3b, spectrum A) which was extremely strong, and distinct from all TSP peaks (spectrum of TSP is shown at 1% concentration in spectrum B). If spermine acts as a free radical scavenger, both upfield and downfield shifts of the 13C spectrum could arise as adducts and, subsequently, fragments are formed. In experiments involving the ROS-generating system, the 13C-NMR peak at 39 ppm, corresponding to enriched spermine, completely disappeared, and two new peaks appeared, one downfield at 60.3 ppm (see below), and one upfield at 19.6 ppm, possibly indicative of a methyl group or shielded methylene moiety (Fig. 3b, spectra C and D). This phenomenon was observed in each experiment at a variety of concentrations of copper chloride and hydrogen peroxide, and at two concentrations of spermine. These data suggest that spermine is converted to at least two adducts in the presence of hydroxyl radical, and support the contention that spermine is capable of acting as a free radical scavenger. The resonance at 60.3 ppm could potentially be attributed to the hydrated form of the dialdehyde (7), described below, and may be formed in the NMR tube in the presence of D2O (this effect is not seen when a spectrum of spermine is acquired in D2O). The adduct producing the signal at 19.6 ppm remains to be identified. Although these NMR signals cannot be used to identify specific adducts between spermine and ROS, they are consistent with direct ROS attack on spermine with consequent adduct formation. Additional experiments are being undertaken to identify the adducts corresponding to these resonances, and to correlate them with the observed mass spectral fragments, as outlined below.
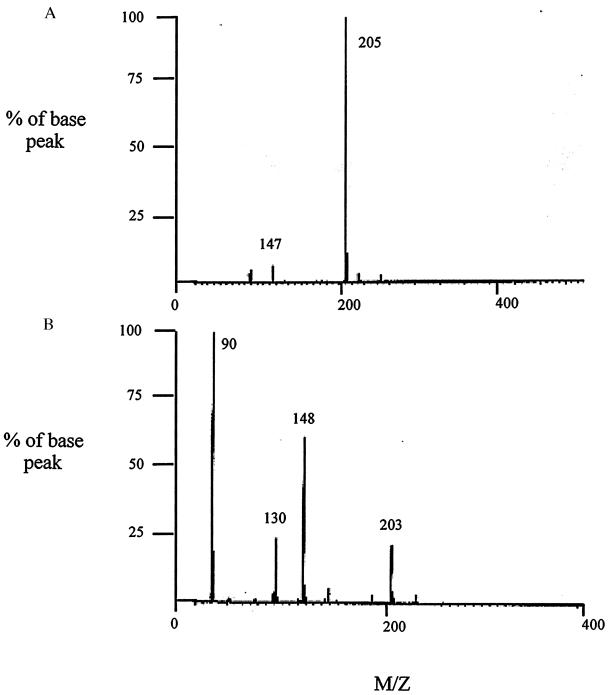
High resolution mass spectrometry was next used to determine the structures of the adducts formed, and to help to elucidate the chemical pathways involved in the reaction between spermine and hydroxyl radicals and other ROS. The reaction mixtures generated as described above were analyzed by chemical ionization (CI) mass spectroscopy (Fig. 4). To determine the low-end sensitivity of the instrument, unlabeled spermine was run as a standard in dilute solution with ammonia, and a recognizable spectrum was obtained with 20 nmol of sample loaded onto the probe tip. Unlabeled spermine produced the expected molecular ion peak at m/z = 203 (data not shown), corresponding to the protonated parent molecule, and produced major peaks at m/z = 146 (loss of one aminopropyl) and m/z = 89 (loss of a second aminopropyl). The CI mass spectrum generated from a dilute solution of bis-α-[13C]spermine (20 nmol of sample) is shown in Fig. 4, spectrum A. A strong molecular ion peak at m/z = 205 resulted from the protonated parent molecule, with a minor peak appearing at m/z 147, corresponding to the loss of a single α-amino[13C]propyl group. The structures of these fragments, as well as their empirical formulas and expected mass spectral peaks, are shown in Table 1.
Figure 4.
Mass spectral analysis for [13C]spermine free radical scavenging experiments. (A) CI spectrum of bis-α-[13C]-enriched spermine standard. (B) CI mass spectral analysis of unknown products of free radical scavenging reaction. Mass spectroscopy was performed on a VG 7250S MicroMass spectrometer (MicroMass Corp., Manchester, U.K.) equipped with a direct chemical ionization (DCI) probe and using ammonia as the reagent gas.
Table 1.
Chemical ionization mass spectral fragments from bis-α-[13C]spermine
| Structure of fragment | Formula | Mr | m/z |
|---|---|---|---|
| 13C212C8H26N4 | 204 | 205 | |
| 13C12C6H19N3 | 146 | 147 | |
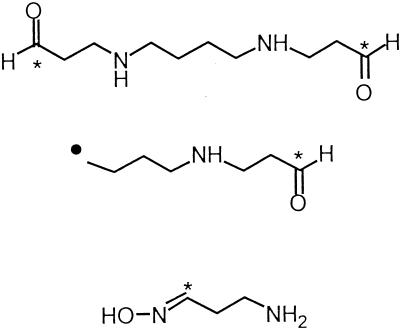
Using the same technique described above, we pooled and lyophilized the reaction mixtures from the free radical scavenging experiments, then subjected them to CI mass spectral analysis. Major peaks appeared at m/z = 203, 148, 130, and 90. Probable identities of the peaks at 203, 130 and, 90 are listed in Table 2, while the identity of the peak at 148 has yet to be determined. Based on the observed fragments from the CI mass spectral studies outlined above, it is postulated that spermine can indeed act as a free radical scavenger in the cell. These results suggest that formation and conversion of the adduct formed from spermine and hydroxy radical are similar to those observed following flavin-containing monooxygenase-mediated N-oxygenation of tyramine (27) and other aliphatic primary amines (28, 29). In the presence of hydroxyl radical, bis-α-[13C]spermine could be expected to form the corresponding bis-N-hydroxyspermine, and then possibly the unstable bis-di-N-hydroxyspermine (5) (Fig. 5). Spontaneous dehydration of (5) would then afford the bis-oxime (6), which would then be readily hydrolyzed to form the dialdehyde (7). While the NMR and mass spectral data suggest that adducts (6) and (7) are the predominant products, additional products or intermediates likely are formed, since there are peaks in each spectrum that clearly do not arise from any of the intermediates in Fig. 5.
Table 2.
Chemical ionization mass spectral fragments from free radical scavenging experiments
| Structure of fragment | Formula | Mr | m/z |
|---|---|---|---|
| 13C212C8H20N2O2 | 202 | 203 | |
| 13C12C6H14NO | 129 | 130 | |
| 13C12C2H8N2O | 89 | 90 | |
Figure 5.
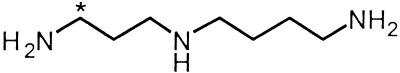
Proposed mechanism for hydroxyl radical scavenging by spermine that is consistent with EPR, NMR, and MS results. Compound (1), bis-α-[13C]spermine; compound (5), bis-α-1-(N,N-dihydroxy)amino-12-(N,N-dihydroxy)amino-4,9-diaza[13C]dodecane; compound (6), 1,12-bis-1,12-di-(hydroxyimino)-4,9-diaza[13C]dodecane; compound (7), 1,12- bis-1,12-dioxo-4,9-diaza[13C]dodecane.
The production of spermine dialdehyde is of potential biological significance. High concentrations of oxidized polyamines can by themselves be toxic (30). However, once spermine is oxidized, its affinity for DNA drops dramatically, thus allowing diffusion away from DNA and making it accessible to the general detoxifying mechanisms of the cell, including reaction with glutathione. Therefore, cellular spermine dialdehyde toxicity as observed by Morgan (30) is not likely to occur as a result of the mechanisms proposed here.
Although the polyamines have been associated with many cellular functions including the regulation of gene expression and programmed cell death (5, 31),they are shown here to also have a potentially critical role as free radical scavengers. Khan et al. (10, 11) first proposed that spermine and spermidine could protect DNA from singlet oxygen damage. They demonstrated DNA damage by singlet oxygen by using pBR322 plasmid DNA gel migration changes (11) and presented evidence that the polyamines were capable of quenching chemically generated singlet oxygen (10). However, no data were provided demonstrating a direct interaction between the polyamines and singlet oxygen. Our results are consistent with the hypothesis of Khan et al. (10, 11) suggesting that polyamines can protect DNA from ROS. Additionally, the current results demonstrate that spermine, a ubiquitous organic polycation, can act directly as a free radical scavenger. It should be noted that many radioprotectants, which presumably act by mechanisms similar to those proposed here, have structures closely related to the polyamines. These structures are so similar, in fact, that some of these agents use the polyamine transporter to enter the cell (32). Taken together, these data suggest that one of the most important functions of spermine may be its role in protecting DNA from ROS damage, subsequent mutation, and potential transformation. Because spermine is closely associated with chromatin in high concentrations, it is well placed to perform this function (7, 8, 14).
Acknowledgments
We thank Dr. James Windak of the University of Michigan Department of Chemistry for aid in the performance of the mass spectral analysis, and S. B. Baylin, K. W. Kinzler, and B. Vogelstein for their helpful comments on the manuscript. This work was supported by National Institutes of Health Grants CA51085, CA58184, ES07141, and CA63552.
ABBREVIATIONS
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- ROS
reactive oxygen species
- CI
chemical ionization
- TSP
sodium 3-(trimethylsilyl)propionate
References
- 1.Tabor C W, Tabor H. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 2.Marton L J, Pegg A E. Annu Rev Pharmacol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 3.Williams K. Biochem J. 1997;325:289–297. doi: 10.1042/bj3250289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park M H, Cooper H L, Folk J E. Proc Natl Acad Sci USA. 1981;78:2869–2873. doi: 10.1073/pnas.78.5.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celano P, Baylin S B, Casero R A. J Biol Chem. 1989;264:8922–8927. [PubMed] [Google Scholar]
- 6.Snyder R D. Biochem J. 1989;260:697–704. doi: 10.1042/bj2600697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feuerstein B G, Pattabiraman N, Marton L J. Nucleic Acids Res. 1990;18:1271–1282. doi: 10.1093/nar/18.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu H S, Marton L J. In: Biological and Therapeutic Implications of the Effects of Polyamines on Chromatin Condensation. Casero R A Jr, editor. Austin, TX: R. G. Landes; 1995. , (5) pp. 101–128. [Google Scholar]
- 9.Brune B, Hartzell P, Nicotera P, Orrenius S. Exp Cell Res. 1991;195:323–329. doi: 10.1016/0014-4827(91)90380-d. [DOI] [PubMed] [Google Scholar]
- 10.Khan A U, Mei Y-H, Wilson T. Proc Natl Acad Sci USA. 1992;89:11426–11427. doi: 10.1073/pnas.89.23.11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan A U, Di Mascio P, Medeiros M H G, Wilson T. Proc Natl Acad Sci USA. 1992;89:11428–11430. doi: 10.1073/pnas.89.23.11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tadolini B. Biochem J. 1988;249:33–36. doi: 10.1042/bj2490033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dypbuk J M, Ankarcrona M, Burkitt M, Sjoholm A, Strom K, Orrenius S, Nicotera P. J Biol Chem. 1994;269:30553–30560. [PubMed] [Google Scholar]
- 14.Lovaas E. Adv Pharmacol. 1997;38:119–149. doi: 10.1016/s1054-3589(08)60982-5. [DOI] [PubMed] [Google Scholar]
- 15.Basu H S, Schwietert H C A, Feuerstein B G, Marton L J. Biochem J. 1990;269:329–334. doi: 10.1042/bj2690329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaiswal A K. Biochem Pharmacology. 1994;48:439–444. doi: 10.1016/0006-2952(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 17.Sarhan S, Seiler N. Biol Chem Hoppe-Seyler. 1989;370:1279–1284. doi: 10.1515/bchm3.1989.370.2.1279. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Kuppusamy P, Zweier J L, Trush M A. Chem Biol Interact. 1995;94:101–120. doi: 10.1016/0009-2797(94)03326-4. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Trush M A. Carcinogenesis. 1993;14:1303–1311. doi: 10.1093/carcin/14.7.1303. [DOI] [PubMed] [Google Scholar]
- 20.Zweier J L, Broderick R, Kuppusamy P, Thompson-Gorman S, Lutty G A. J Biol Chem. 1994;269:24156–24162. [PubMed] [Google Scholar]
- 21.Edwards M L, Stemerick D M, Bitonti A J, Dumont J A, McCaan P P, Bey P, Sjoerdsma A. J Med Chem. 1991;34:569–574. doi: 10.1021/jm00106a015. [DOI] [PubMed] [Google Scholar]
- 22.Rylander P N. In: Hydrogenation Methods. Rylander P N, editor. New York: Academic; 1985. pp. 95–99. [Google Scholar]
- 23.Bergeron R J, Garlich J R, Stolowich N J. J Org Chem. 1984;49:2997–3001. [Google Scholar]
- 24.Sagripanti J-L, Kraemer K H. J Biol Chem. 1989;264:1729–1734. [PubMed] [Google Scholar]
- 25.Kramer D, Stanek J, Diegelman P, Regenass U, Schneider P, Porter C W. Biochem Pharmacol. 1995;50:1433–1443. doi: 10.1016/0006-2952(95)02037-3. [DOI] [PubMed] [Google Scholar]
- 26.Hanna P M, Chamulitrat W, Mason R P. Arch Biochem Biophys. 1992;296:640–644. doi: 10.1016/0003-9861(92)90620-c. [DOI] [PubMed] [Google Scholar]
- 27.Hanna P M, Mason R P. Arch Biochem Biophys. 1992;295:205–213. doi: 10.1016/0003-9861(92)90507-s. [DOI] [PubMed] [Google Scholar]
- 28.Lin J, Cashman J R. Chem Res Toxicol. 1997;10:842–852. doi: 10.1021/tx970030o. [DOI] [PubMed] [Google Scholar]
- 29.Lin J, Berkman C E, Cashman J R. Chem Res Toxicol. 1996;9:1183–1193. doi: 10.1021/tx9600614. [DOI] [PubMed] [Google Scholar]
- 30.Morgan D M L. Biochem J. 1987;242:347–352. doi: 10.1042/bj2420347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ha H C, Woster P M, Yager J D, Casero R A., Jr Proc Natl Acad Sci USA. 1997;94:11557–11562. doi: 10.1073/pnas.94.21.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell J L, Judd G G, Diveley R R, Choe C Y, Leyser A. Carcinogenesis. 1995;16:3063–3068. doi: 10.1093/carcin/16.12.3063. [DOI] [PubMed] [Google Scholar]