Abstract
The active transport of glutamine by Escherichia coli occurs via a single osmotic shock-sensitive transport system which is known to be dependent upon a periplasmic binding protein specific for glutamine. We obtained a mutant that had elevated levels of glutamine transport and overproduced the glutamine binding protein. From this strain many point mutants and deletion-carrying strains defective in glutamine transport were isolated by a variety of techniques. The genetic locus coding for the glutamine transport system, glnP, and the regulatory mutation which causes overproduction of the transport system were both shown to map at 17.7 min on the E. coli chromosome, and it was demonstrated that the glnP locus contains the structural gene for the glutamine binding protein. Evidence was also obtained that the glutamine transport system, by an unknown mechanism, plays a direct role in the catabolism of glutamate and, hence, of glutamine and proline as well.
Full text
PDF




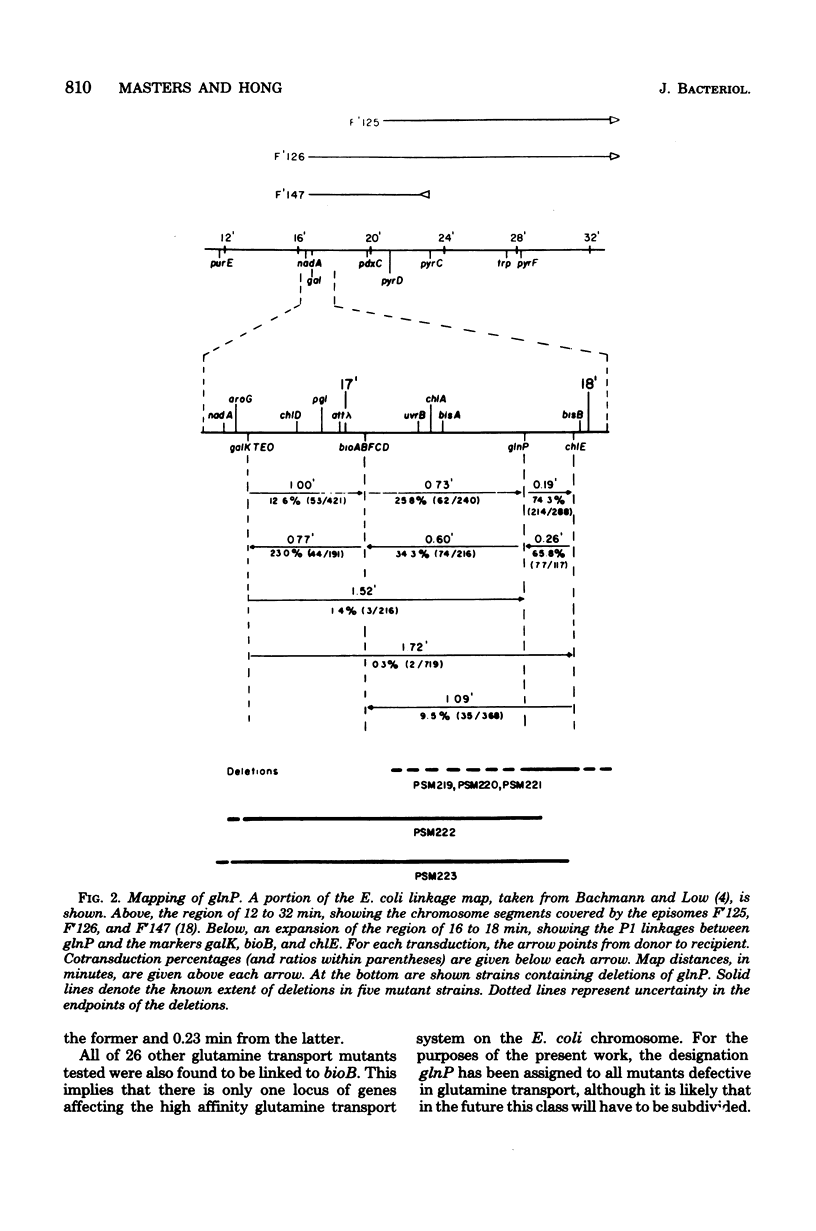


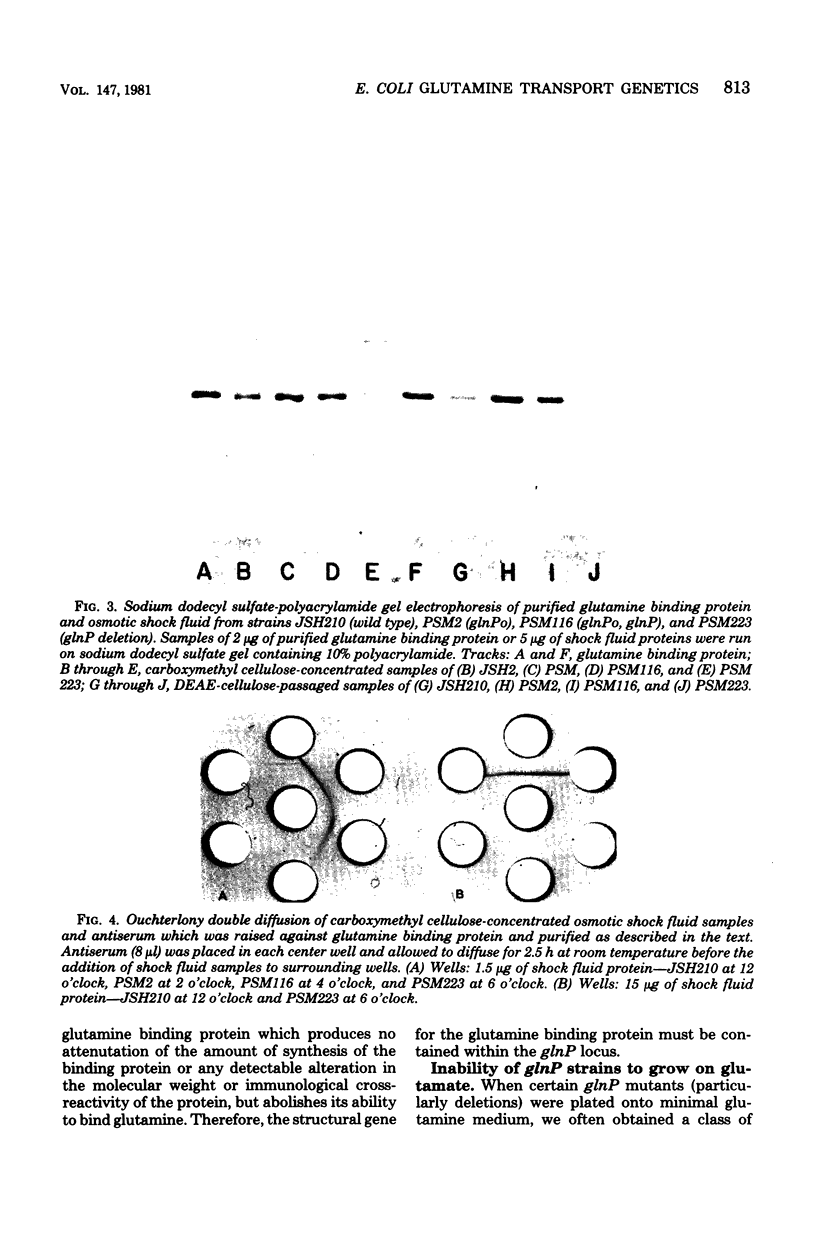

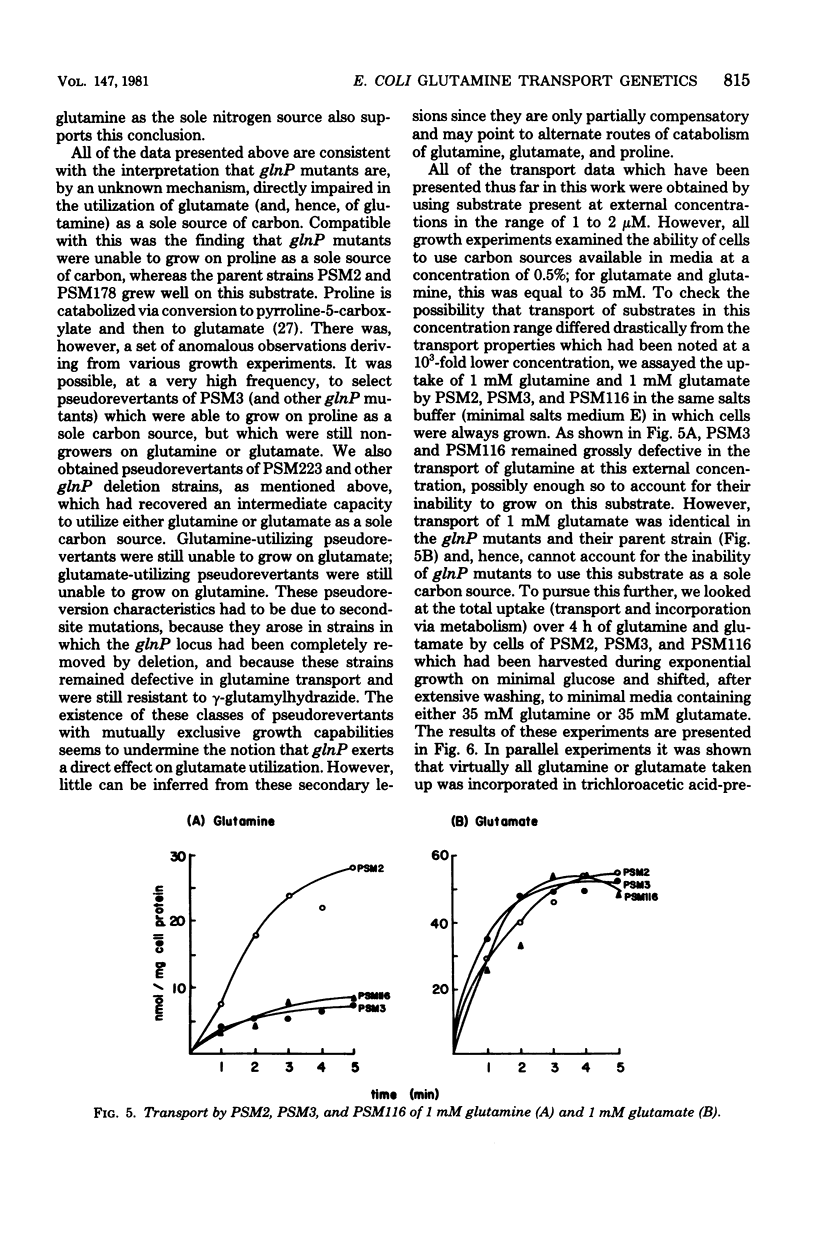




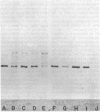
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Cleary P., Campbell A. A deletion analysis of prophage lambda and adjacent genetic regions. Proc Natl Acad Sci U S A. 1968 Nov;61(3):956–962. doi: 10.1073/pnas.61.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1096–1103. doi: 10.1073/pnas.66.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Betteridge P. R., Ayling P. D. The role of methionine transport-defective mutations in resistance to methionine sulphoximine in Salmonella typhimurium. Mol Gen Genet. 1975;138(1):41–52. doi: 10.1007/BF00268826. [DOI] [PubMed] [Google Scholar]
- Cleary P. P., Campbell A. Deletion and complementation analysis of biotin gene cluster of Escherichia coli. J Bacteriol. 1972 Nov;112(2):830–839. doi: 10.1128/jb.112.2.830-839.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick D., Calvo J. M., Klopotowski T., Ames B. N. Compounds which serve as the sole source of carbon or nitrogen for Salmonella typhimurium LT-2. J Bacteriol. 1969 Oct;100(1):215–219. doi: 10.1128/jb.100.1.215-219.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Adler J. Role of the galactose binding protein in chemotaxis of Escherichia coli toward galactose. Nat New Biol. 1971 Mar 24;230(12):101–104. doi: 10.1038/newbio230101a0. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Foulds J., Soll L., Berg P. Instability of a missense suppressor resulting from a duplication of genetic material. J Mol Biol. 1969 Feb 14;39(3):563–581. doi: 10.1016/0022-2836(69)90146-6. [DOI] [PubMed] [Google Scholar]
- Kreishman G. P., Robertson D. E., Ho C. PMR studies of the substrate induced conformational change of glutamine binding protein from E. coli. Biochem Biophys Res Commun. 1973 Jul 2;53(1):18–23. doi: 10.1016/0006-291x(73)91394-6. [DOI] [PubMed] [Google Scholar]
- Kustu S. G., McFarland N. C., Hui S. P., Esmon B., Ames G. F. Nitrogen control of Salmonella typhimurium: co-regulation of synthesis of glutamine synthetase and amino acid transport systems. J Bacteriol. 1979 Apr;138(1):218–234. doi: 10.1128/jb.138.1.218-234.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lever J. E. Quantitative assay of the binding of small molecules to protein: comparison of dialysis and membrane filter assays. Anal Biochem. 1972 Nov;50(1):73–83. doi: 10.1016/0003-2697(72)90487-3. [DOI] [PubMed] [Google Scholar]
- Lieberman M. A., Hong J. S. A mutant of Escherichia coli defective in the coupling of metabolic energy to active transport. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4395–4399. doi: 10.1073/pnas.71.11.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. The metabolic pathway of glutamate in Escherichia coli K-12. Biochim Biophys Acta. 1969 Apr 1;177(2):314–320. doi: 10.1016/0304-4165(69)90141-x. [DOI] [PubMed] [Google Scholar]
- Marty B., Gaudin C., Ragot M., Belaich A., Belaích J. P. Microcalorimetric study of glutamine fixation on the glutamine-binding protein of Escherichia coli. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1118–1125. doi: 10.1016/0006-291x(79)90233-x. [DOI] [PubMed] [Google Scholar]
- Oeschger M. P., Berlyn M. K. A simple procedure for localized mutagenesis using nitrosoguanidine. Mol Gen Genet. 1974;134(1):77–83. doi: 10.1007/BF00332814. [DOI] [PubMed] [Google Scholar]
- Prusiner S., Miller R. E., Valentine R. C. Adenosine 3':5'-cyclic monophosphate control of the enzymes of glutamine metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2922–2926. doi: 10.1073/pnas.69.10.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzkin B., Roth J. Cluster of genes controlling proline degradation in Salmonella typhimurium. J Bacteriol. 1978 Feb;133(2):744–754. doi: 10.1128/jb.133.2.744-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve E. C., Doherty P. Linkage relationships of two genes causing partial resistance to chloramphenicol in Escherichia coli. J Bacteriol. 1968 Oct;96(4):1450–1451. doi: 10.1128/jb.96.4.1450-1451.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. L. Transduction studies on the relation between prophage and host chromosome. J Mol Biol. 1965 Jul;12(3):892–912. doi: 10.1016/s0022-2836(65)80336-9. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Schechter B., Sela M. Combining sites of antibodies with L-alanine and D-alanine peptide specificity and the effect of serum proteolytic activity on their estimation. Biochim Biophys Acta. 1966 Oct 31;127(2):438–456. doi: 10.1016/0304-4165(66)90398-9. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Furlong C. E. Resolution of the multiplicity of the glutamate and aspartate transport systems of Escherichia coli. J Biol Chem. 1977 Dec 25;252(24):9055–9064. [PubMed] [Google Scholar]
- Schroeder D. D., Allison A. J., Buchanan J. M. Biosynthesis of the purines. XXXII. Effect of albizziin and other reagents on the activity of formylglycinamide ribonucleotide amidotransferase. J Biol Chem. 1969 Nov 10;244(21):5856–5865. [PubMed] [Google Scholar]
- Spencer M. E., Lebeter V. M., Guest J. R. Location of the Aspartase Gene (aspA) on the linkage map of Escherichia coli K12. J Gen Microbiol. 1976 Nov;97(1):73–82. doi: 10.1099/00221287-97-1-73. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Yamaguchi J., Tokushige M. Studies on aspartase. I. Purification and molecular properties of aspartase from Escherichia coli. Biochim Biophys Acta. 1973 Sep 15;321(1):369–381. doi: 10.1016/0005-2744(73)90092-2. [DOI] [PubMed] [Google Scholar]
- Tosa T., Sato T., Nishida Y., Chibata I. Reason for higher stability of aspartase activity of immobilized Escherichia coli cells. Biochim Biophys Acta. 1977 Jul 8;483(1):193–202. doi: 10.1016/0005-2744(77)90021-3. [DOI] [PubMed] [Google Scholar]
- Urm E., Leisinger T., Vogel H. J. Magnesium sensitivity of L-aspartate: 2-oxoglutarate aminotransferase in Escherichia coli. Biochim Biophys Acta. 1973 Apr 12;302(2):249–260. doi: 10.1016/0005-2744(73)90154-x. [DOI] [PubMed] [Google Scholar]
- VENDER J., RICKENBERG H. V. AMMONIA METABOLISM IN A MUTANT OF ESCHERICHIA COLI LACKING GLUTAMATE DEHYDROGENASE. Biochim Biophys Acta. 1964 Jul 15;90:218–220. doi: 10.1016/0304-4165(64)90149-7. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Venables W. A., Guest J. R. Transduction of nitrate reductase loci of Escherichia coli by phages P-1 and lambda. Mol Gen Genet. 1968;103(2):127–140. doi: 10.1007/BF00427140. [DOI] [PubMed] [Google Scholar]
- Weiner J. H., Furlong C. E., Heppel L. A. A binding protein for L-glutamine and its relation to active transport in E. coli. Arch Biochem Biophys. 1971 Feb;142(2):715–717. doi: 10.1016/0003-9861(71)90538-8. [DOI] [PubMed] [Google Scholar]
- Willis R. C., Furlong C. E. Purification and properties of a periplasmic glutamate-aspartate binding protein from Escherichia coli K12 strain W3092. J Biol Chem. 1975 Apr 10;250(7):2574–2580. [PubMed] [Google Scholar]
- Willis R. C., Iwata K. K., Furlong C. E. Regulation of Glutamine Transport in Escherichia coli. J Bacteriol. 1975 Jun;122(3):1032–1037. doi: 10.1128/jb.122.3.1032-1037.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis R. C., Seegmiller J. E. A filtration assay specific for the determination of small quantities of L-glutamine. Anal Biochem. 1976 May 7;72:66–77. doi: 10.1016/0003-2697(76)90507-8. [DOI] [PubMed] [Google Scholar]