Abstract
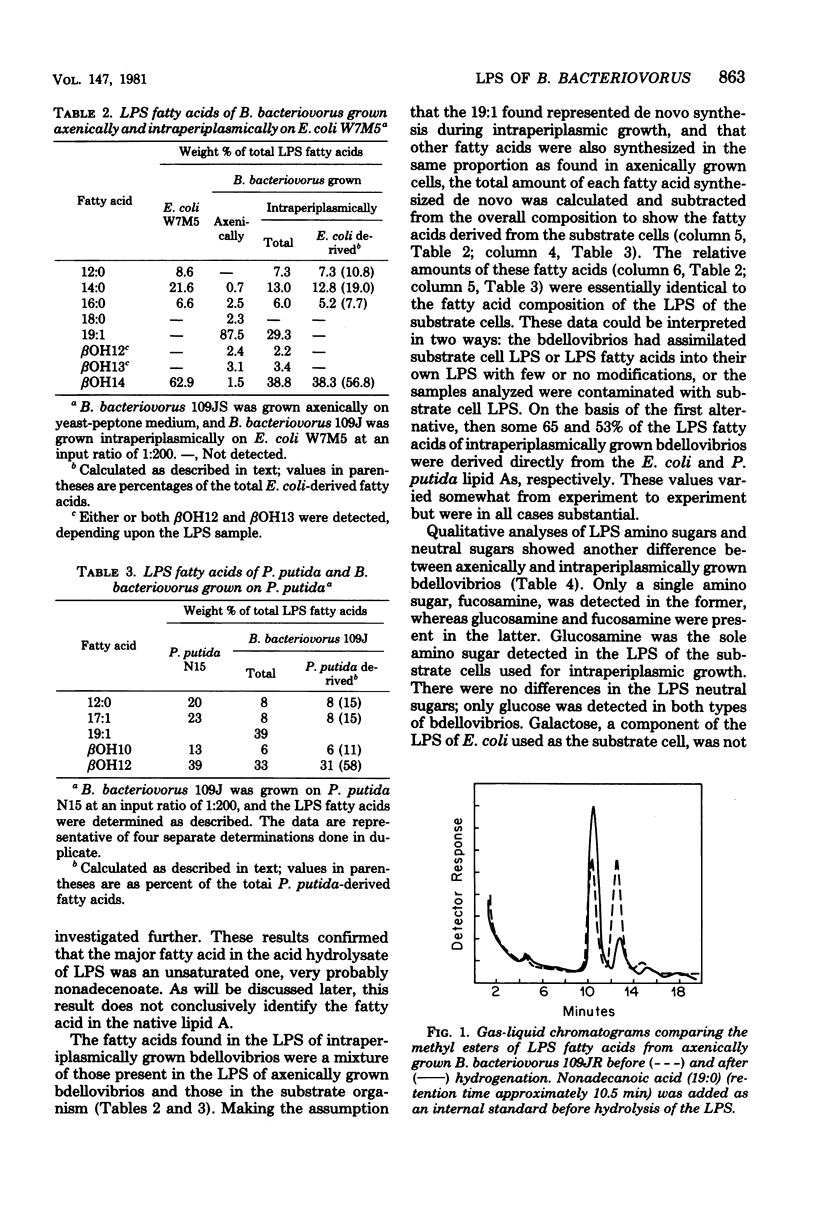
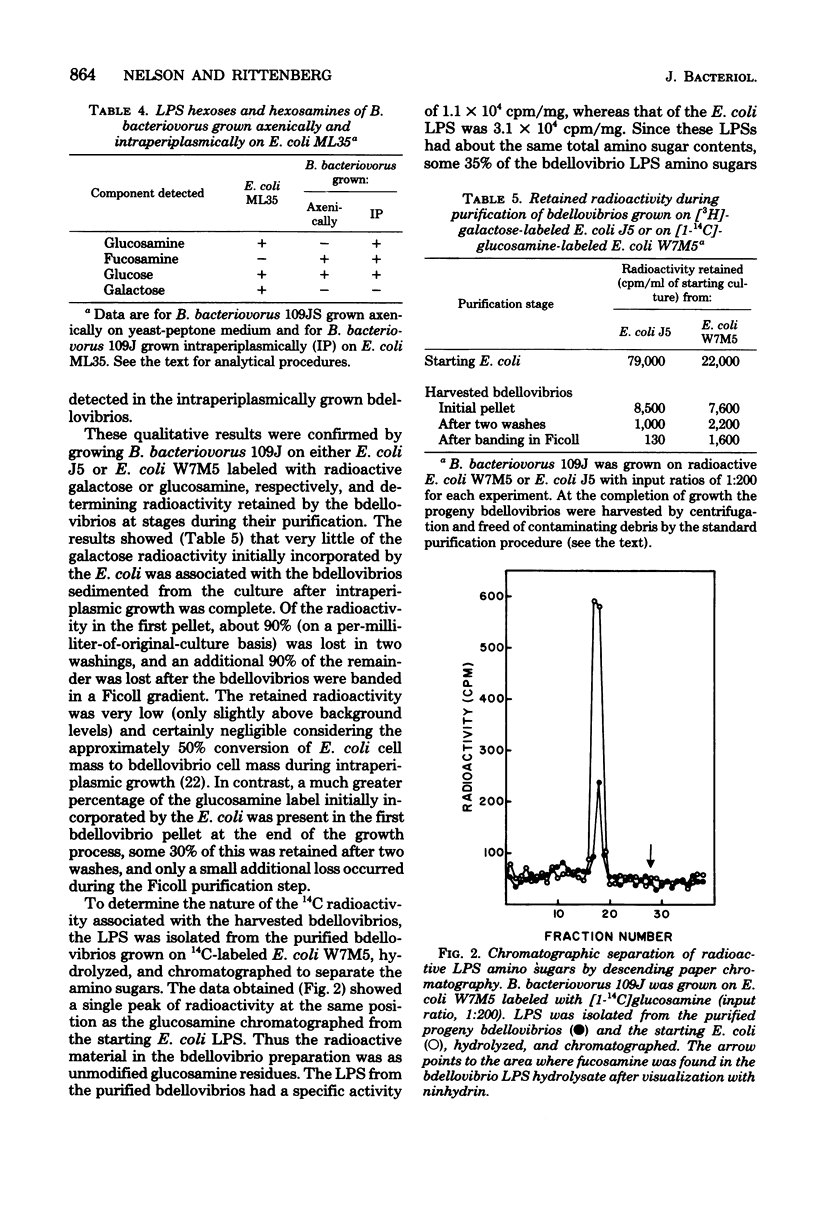
The composition of Bdellovibrio bacteriovorus lipopolysaccharide (LPS) was determined for cells grown axenically and intraperiplasmically on Escherichia coli or Pseudomonas putida. The LPS of axenically grown bdellovibrios contained glucose and fucosamine as the only detectable neutral sugar and amino sugar, and nonadecenoic acid (19:1) as the predominant fatty acid. Additional fatty acids, heptose, ketodeoxyoctoic acid, and phosphate were also detected. LPS from bdellovibrios grown intraperiplasmically contained components characteristic of both axenically grown bdellovibrios and the substrate cells. Substrate cell-derived LPS fatty acids made up the majority of the bdellovibrio LPS fatty acids and were present in about the same proportions as in the substrate cell LPS. Glucosamine derived from E. coli LPS amounted to about one-third of the hexosamine residues in intraperiplasmically grown bdellovibrio LPS. However, galactose, characteristic of the E. coli outer core and O antigen, was not detected in the bdellovibrio LPS, suggesting that only lipid A components of the substrate cell were incorporated. Substrate cell-derived and bdellovibrio-synthesized LPS materials were conserved in the B. bacteriovorus outer membrane for at least two cycles of intraperiplasmic growth. When bdellovibrios were grown on two different substrate cells successively, lipid A components were taken up from the second while the components incorporated from the lipid A of the first were conserved in the bdellovibrio LPS. The data show that substrate cell lipid A components were incorporated into B. bacteriovorus lipid A during intraperiplasmic growth with little or no change, and that these components, fatty acids and hexosamines, comprised a substantial portion of bdellovibrio lipid A.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Burnham J. C., Hashimoto T., Conti S. F. Ultrastructure and cell division of a facultatively parasitic strain of Bdellovibrio bacteriovorus. J Bacteriol. 1970 Mar;101(3):997–1004. doi: 10.1128/jb.101.3.997-1004.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Drewry D. T., Symes K. C., Gray G. W., Wilkinson S. G. Studies of polysaccharide fractions from the lipopolysaccharide of Pseudomonas aeruginosa N.C.T.C. 1999. Biochem J. 1975 Jul;149(1):93–106. doi: 10.1042/bj1490093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbert R. E., Weckesser J., Tharanathan R. N., Mayer H. Isolation and characterization of the lipopolysaccharide of Thiocapsa roseopersicina. Eur J Biochem. 1978 Oct;90(2):241–246. doi: 10.1111/j.1432-1033.1978.tb12596.x. [DOI] [PubMed] [Google Scholar]
- Jarrell K., Kropinski A. M. The chemical composition of the lipopolysaccharide from Pseudomonas aeruginosa strain PAO and a spontaneously derived rough mutant. Microbios. 1977;19(76):103–116. [PubMed] [Google Scholar]
- Kuenen J. G., Rittenberg S. C. Incorporation of long-chain fatty acids of the substrate organism by Bdellovibrio bacteriovorus during intraperiplasmic growth. J Bacteriol. 1975 Mar;121(3):1145–1157. doi: 10.1128/jb.121.3.1145-1157.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Domains involving nonrandom distribution of lipopolysaccharide in the outer membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5065–5068. doi: 10.1073/pnas.74.11.5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Rittenberg S. C. Kinetics of deoxyribonucleic acid destruction and synthesis during growth of Bdellovibrio bacteriovorus strain 109D on pseudomonas putida and escherichia coli. J Bacteriol. 1972 Sep;111(3):664–673. doi: 10.1128/jb.111.3.664-673.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J. J., Stewart J. C. Structural studies on the lipopolysaccharide of a rough strain of Escherichia coli. Eur J Biochem. 1972 Sep 18;29(2):308–318. doi: 10.1111/j.1432-1033.1972.tb01990.x. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Rittenberg S. C. Partial characterization of lipid A of intraperiplasmically grown Bdellovibrio bacteriovorus. J Bacteriol. 1981 Sep;147(3):869–874. doi: 10.1128/jb.147.3.869-874.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard M. A., Langley D., Rittenberg S. Effects of methotrexate on intraperiplasmic and axenic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1131–1136. doi: 10.1128/jb.121.3.1131-1136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Rittenberg S. C., Hespell R. B. Energy efficiency of intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1158–1165. doi: 10.1128/jb.121.3.1158-1165.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C., Langley D. Utilization of nucleoside monophosphates per Se for intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1137–1144. doi: 10.1128/jb.121.3.1137-1144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C., Shilo M. Early host damage in the infection cycle of Bdellovibrio bacteriovorus. J Bacteriol. 1970 Apr;102(1):149–160. doi: 10.1128/jb.102.1.149-160.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney S. A., Goldfine H., Sweeley C. C. The identification of trans-2-tetradecenoic acid in hydrolysates of lipid A from Escherichia coli. Biochim Biophys Acta. 1972 Jul 7;270(3):289–295. doi: 10.1016/0005-2760(72)90192-0. [DOI] [PubMed] [Google Scholar]
- Rosson R. A., Rittenberg S. C. Regulated breakdown of Escherichia coli deoxyribonucleic acid during intraperiplasmic growth of Bdellovibrio bacteriovorus 109J. J Bacteriol. 1979 Nov;140(2):620–633. doi: 10.1128/jb.140.2.620-633.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- Smith P. F., Langworthy T. A., Mayberry W. R. Distribution and composition of lipopolysaccharides from mycoplasmas. J Bacteriol. 1976 Mar;125(3):916–922. doi: 10.1128/jb.125.3.916-922.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Rittenberg S. C. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: solubilization of Escherichia coli peptidoglycan. J Bacteriol. 1978 Sep;135(3):998–1007. doi: 10.1128/jb.135.3.998-1007.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon M., Shil M. Interacton of Bdellovibrio bacteriovorus and host bacteria. I. Kinetic studies of attachment and invasion of Escherichia coli B by Bdellovibrio bacteriovorus. J Bacteriol. 1968 Mar;95(3):744–753. doi: 10.1128/jb.95.3.744-753.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- Weckesser J., Drews G., Roppel J., Mayer H., Fromme I. The lipopolysaccharides (O-antigens) of Rhodopseudomonas viridis. Arch Microbiol. 1974;101(3):233–245. doi: 10.1007/BF00455941. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G., Galbrath L. Studies of lipopolysaccharides from Pseudomonas aeruginosa. Eur J Biochem. 1975 Mar 17;52(2):331–343. doi: 10.1111/j.1432-1033.1975.tb04001.x. [DOI] [PubMed] [Google Scholar]


