Abstract
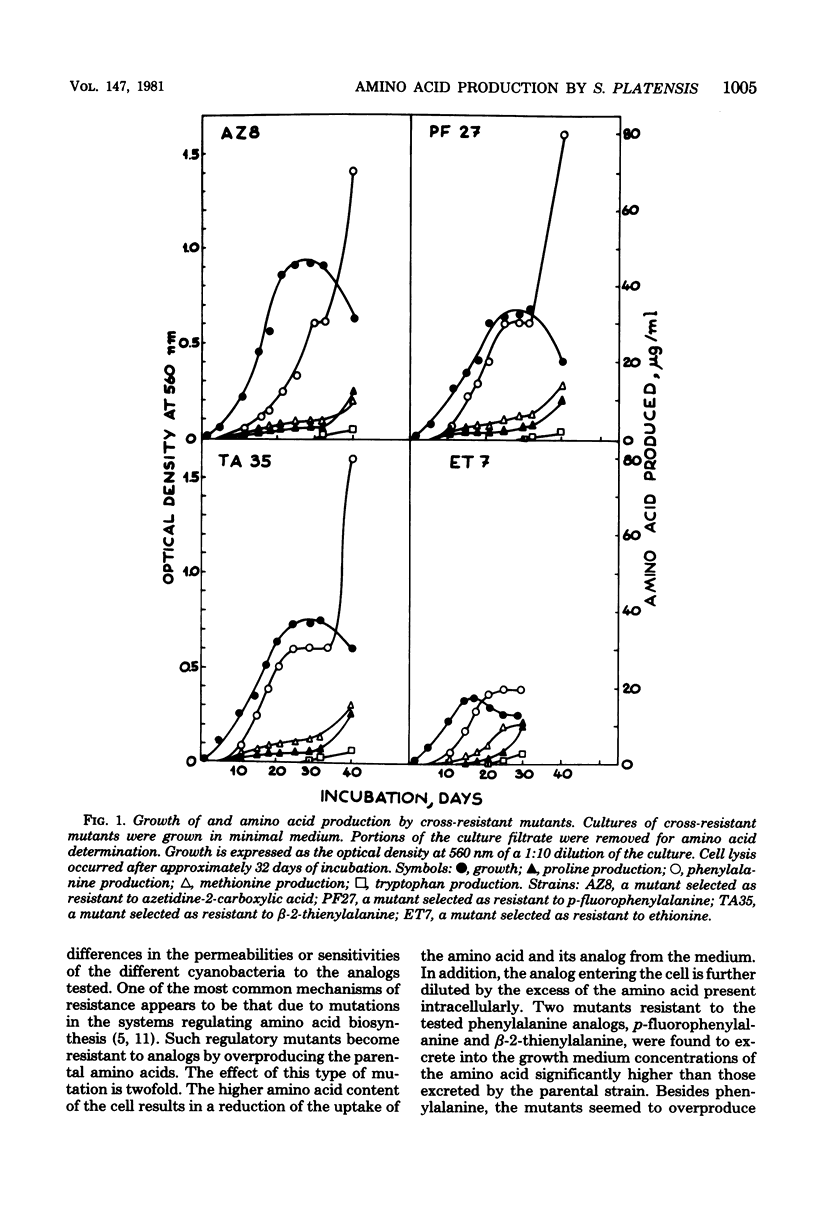
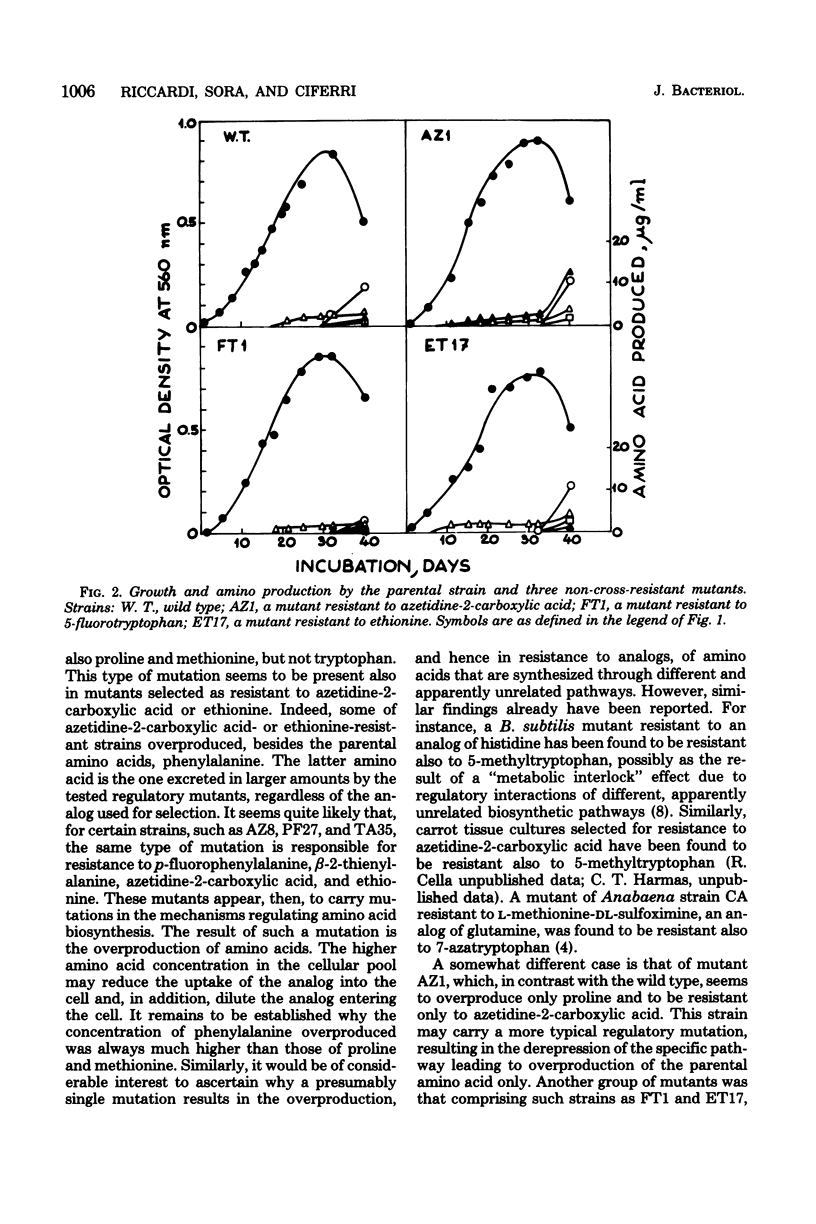
Mutants of Spirulina platensis resistant to 5-fluorotryptophan, beta-3-thienyl-alanine, ethionine, p-fluorophenylalanine, or azetidine-2-carboxylic acid were isolated. Some of these mutants appeared to be resistant to more than one analog and to overproduce the corresponding amino acids. A second group was composed of mutants that were resistant to one analog only. Of the latter mutants, one resistant to azetidine-2-carboxylic acid was found to overproduce proline only, whereas one resistant to fluorotryptophan and one resistant to ethionine did not overproduce any of the tested amino acids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden L., Lewis D., Tristram H. Toxic amino acids: their action as antimetabolites. Adv Enzymol Relat Areas Mol Biol. 1967;29:89–163. doi: 10.1002/9780470122747.ch3. [DOI] [PubMed] [Google Scholar]
- Gotto J. W., Tabita F. R., Van Baalen C. Mutants of Anabaena strain CA altered in their ability to grow under nitrogen-fixing conditions. J Bacteriol. 1979 Nov;140(2):327–332. doi: 10.1128/jb.140.2.327-332.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G., Flick M. B., Jensen R. A. Approach to recognition of regulatory mutants of cyanobacteria. J Bacteriol. 1980 Aug;143(2):981–988. doi: 10.1128/jb.143.2.981-988.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzclaw W. D., Chapman L. F., Gowans C. S. Some regulatory properties of phospho-2-keto-3-deoxyheptonate aldolase from the blue-green alga Anacystis nidulans. Biochim Biophys Acta. 1972 May 12;268(2):562–572. doi: 10.1016/0005-2744(72)90353-1. [DOI] [PubMed] [Google Scholar]
- Jensen R. A. Metabolic interlock. Regulatory interactions exerted between biochemical pathways. J Biol Chem. 1969 Jun 10;244(11):2816–2823. [PubMed] [Google Scholar]
- Neihardt F. C., Parker J., McKeever W. G. Function and regulation of aminoacyl-tRNA synthetases in prokaryotic and eukaryotic cells. Annu Rev Microbiol. 1975;29:215–250. doi: 10.1146/annurev.mi.29.100175.001243. [DOI] [PubMed] [Google Scholar]
- Phares W., Chapman L. F. Anacystis nidulans mutants resistant to aromatic amino acid analogues. J Bacteriol. 1975 Jun;122(3):943–948. doi: 10.1128/jb.122.3.943-948.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Metabolite analogs as genetic and biochemical probes. Adv Genet. 1971;16:119–140. doi: 10.1016/s0065-2660(08)60356-9. [DOI] [PubMed] [Google Scholar]
- Yadava P. K. Histidine sensitive variant of the blue-green alga Nostoc muscorum: response to corepressors of histidine biosynthesis. Mol Gen Genet. 1979 Feb 16;170(1):109–111. doi: 10.1007/BF00268586. [DOI] [PubMed] [Google Scholar]


