Abstract
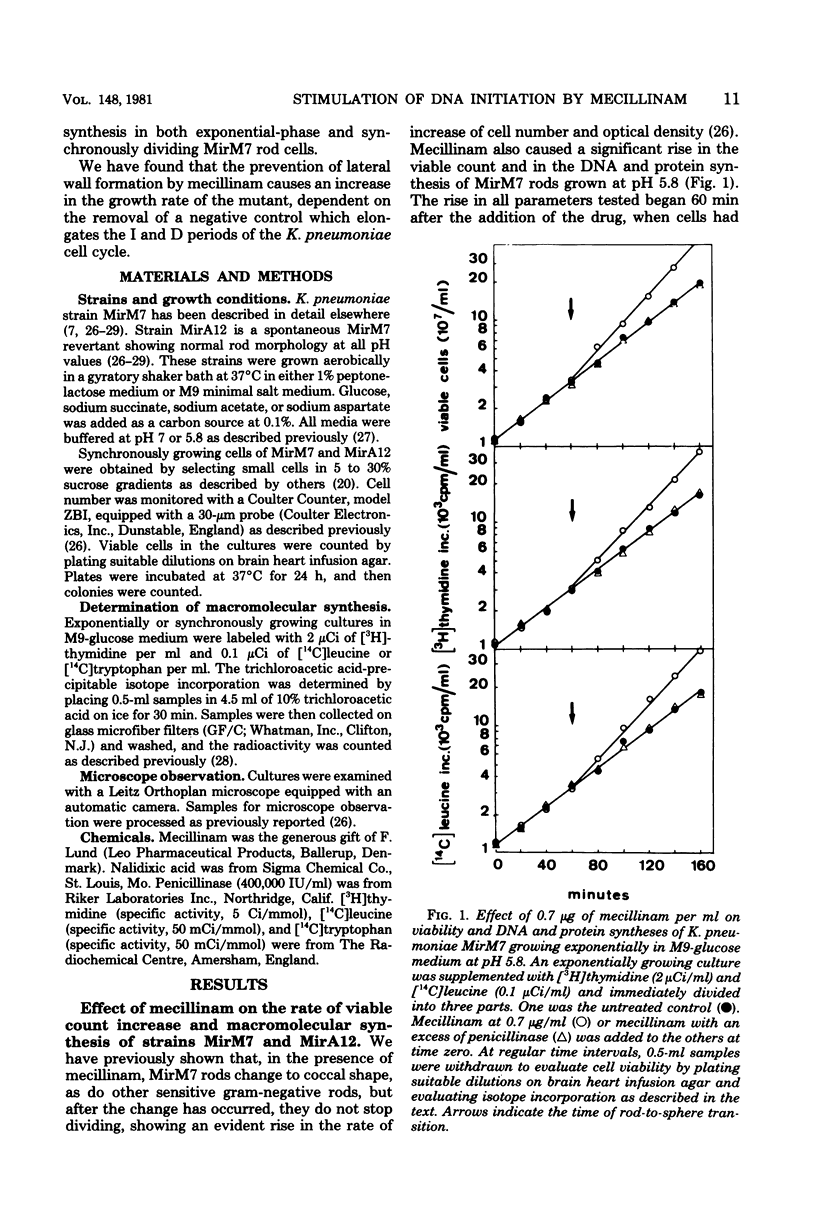
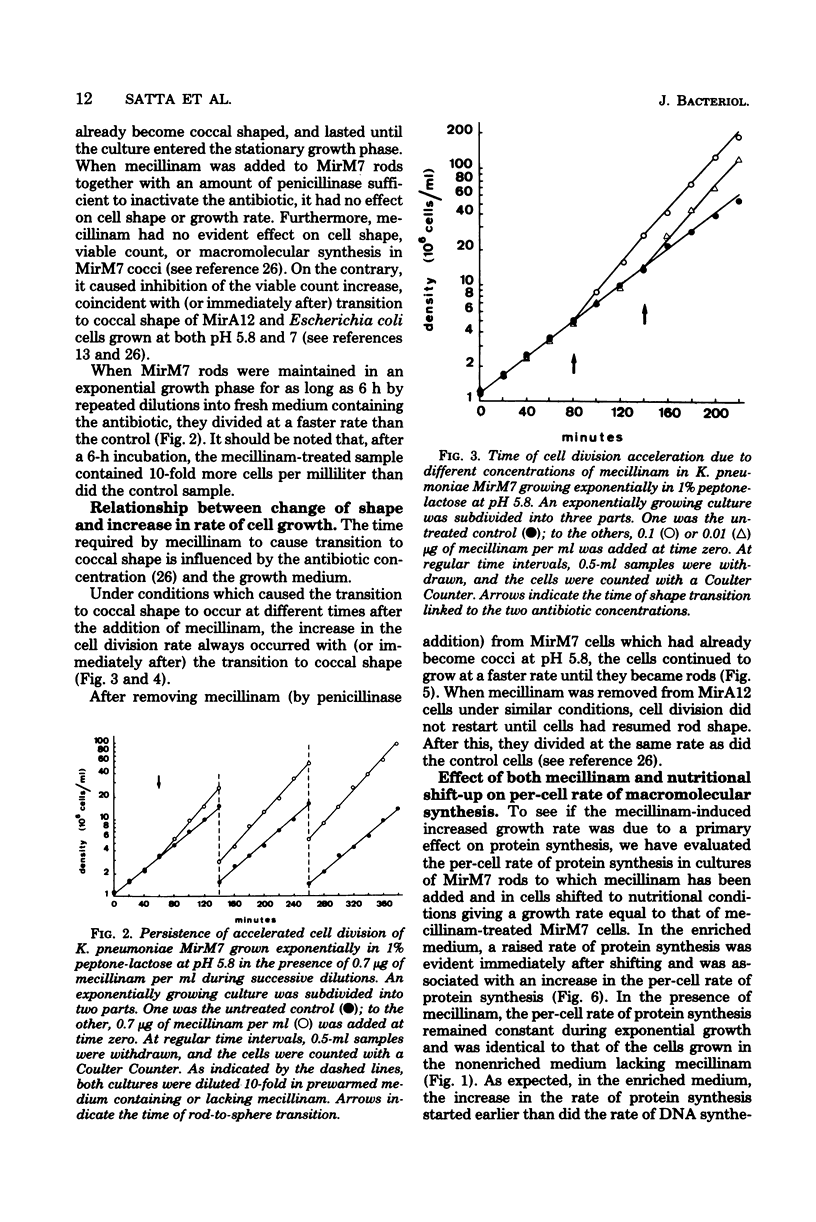
The effects of mecillinam on the growth of rods of the pH-conditional morphology mutant MirM7 was studied. It has been found that mecillinam causes, coincident with transition to coccal shape, a balanced rise in the rate of viable count increase and the rate of macromolecular synthesis which lasts either until the cells enter a stationary growth phase or indefinitely, in the case of continuously diluted cultures. When the antibiotic is removed from cells which have already become coccoid, cells continue to grow at a faster rate until they resume the rod shape. No change in the per-cell rate of protein synthesis has been seen in untreated or mecillinam-treated cells before or after the change in growth rate. Studies with synchronously growing cells have shown that the antibiotic causes a shortening in the I period (initiation of deoxyribonucleic acid replication). Evaluation of the residual divisions in nalidixic acid-treated, exponential-phase cells has shown that mecillinam also shortens the D period (cell division). It is proposed that, in strain MirM7, inhibition of lateral wall elongation by the antibiotic allows the initiation of a new septum, though inhibition is still in progress. The initiation of a new septum is, in turn, responsible for both the early inibition of deoxyribonucleic acid replication and accelerated division. In the parental strain, MirA12, as well as in other sensitive gram-negative rods which divide, become cocci, and stop dividing after addition of the antibiotic, inhibition of lateral wall formation activates a feedback mechanism which prevents insertion of new septa (Satta et al., J. Bacteriol. 142:43-51, 1980). Consequently, no early initiation of deoxyribonucleic acid replication is observed, and the last division allowed by the antibiotic occurs in due time. This negative control is missing in MirM7.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. G., Smith J. A., Knudsen R. C., Walker J. R. Initial characterization of temperature-sensitive cell division mutants of Escherichia coli. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1074–1079. doi: 10.1016/0006-291x(72)90943-6. [DOI] [PubMed] [Google Scholar]
- Donachie W. D. Relationship between cell size and time of initiation of DNA replication. Nature. 1968 Sep 7;219(5158):1077–1079. doi: 10.1038/2191077a0. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Fontana R., Canepari P., Satta G. Alterations in peptidoglycan chemical composition associated with rod-to-sphere transition in a conditional mutant of Klebsiella pneumoniae. J Bacteriol. 1979 Sep;139(3):1028–1038. doi: 10.1128/jb.139.3.1028-1038.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., James R., Paradee A. B. Evidence of the involvement of an outer membrane protein in DNA initiation. J Biol Chem. 1976 Jun 10;251(11):3470–3479. [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Deoxyribonucleic acid synthesis during the division cycle of Escherichia coli: a comparison of strains B-r, K-12, 15, and 15T- under conditions of slow growth. J Bacteriol. 1974 Mar;117(3):1216–1223. doi: 10.1128/jb.117.3.1216-1223.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E. Initiation of chromosome replication in Escherichia coli. II. Analysis of the control mechanism. J Mol Biol. 1974 Mar 25;84(1):21–36. doi: 10.1016/0022-2836(74)90210-1. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E., Pierucci O. DNA synthesis during the division cycle of three substrains of Escherichia coli B/r. J Mol Biol. 1976 Apr 15;102(3):477–486. doi: 10.1016/0022-2836(76)90329-6. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L., Boothby D., Shockman G. D. Effect of inhibition of deoxyribonucleic acid and protein synthesis on the direction of cell wall growth in Streptococcus faecalis. J Bacteriol. 1974 May;118(2):681–692. doi: 10.1128/jb.118.2.681-692.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- James R., Haga J. Y., Pardee A. B. Inhibition of an early event in the cell division cycle of Escherichia coli by FL1060, an amidinopenicillanic acid. J Bacteriol. 1975 Jun;122(3):1283–1292. doi: 10.1128/jb.122.3.1283-1292.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz P. J., Schaechter M. The attachment of the bacterial chromosome to the cell membrane. Int Rev Cytol. 1975;41:1–28. doi: 10.1016/s0074-7696(08)60964-x. [DOI] [PubMed] [Google Scholar]
- Marvin D. A. Control of DNA replication by membrane. Nature. 1968 Aug 3;219(5153):485–486. doi: 10.1038/219485a0. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Penicillin-induced secretion of soluble, uncross-linked peptidoglycan by Micrococcus luteus cells. Biochemistry. 1974 Nov 19;13(24):5045–5053. doi: 10.1021/bi00721a028. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Schwarz U. Regulation of murein biosynthesis and septum formation in filamentous cells of Escherichia coli PAT 84. J Bacteriol. 1977 Mar;129(3):1593–1600. doi: 10.1128/jb.129.3.1593-1600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. T., Burman L. FL-1060: a new penicillin with a unique mode of action. Biochem Biophys Res Commun. 1973 Apr 16;51(4):863–868. doi: 10.1016/0006-291x(73)90006-5. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Effet des sels et autres composés sur le phénotype de mutants thermosensibles de Escherichia coli. Ann Microbiol (Paris) 1973 Jan;124(1):29–43. [PubMed] [Google Scholar]
- Rogers H. J., McConnell M., Burdett I. D. Cell wall or membrane mutants of Bacillus subtilis and Bacillus licheniformis with grossly deformed morphology. Nature. 1968 Jul 20;219(5151):285–288. doi: 10.1038/219285a0. [DOI] [PubMed] [Google Scholar]
- Satta G., Canepari P., Botta G., Fontana R. Control of cell septation by lateral wall extension in a pH-conditional morphology mutant of Klebsiella pneumoniae. J Bacteriol. 1980 Apr;142(1):43–51. doi: 10.1128/jb.142.1.43-51.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Fontana R., Canepari P., Botta G. Peptidoglycan synthesis in cocci and rods of a pH-dependent, morphologically conditional mutant of Klebsiella pneumoniae. J Bacteriol. 1979 Feb;137(2):727–734. doi: 10.1128/jb.137.2.727-734.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Fontana R. Characterization of a conditional mutant with altered envelope showing pH-dependent morphology and temperature-dependent division. J Gen Microbiol. 1974 Jan;80(1):51–63. doi: 10.1099/00221287-80-1-51. [DOI] [PubMed] [Google Scholar]
- Satta G., Pruzzo C., Debbia E., Fontana R. Close association between shape alteration and loss of immunity to superinfection in a wild-type Klebsiella pneumoniae stable lysogen which can be both immune and nonimmune to superinfection. J Virol. 1978 Dec;28(3):772–785. doi: 10.1128/jvi.28.3.772-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M., Schaechter M. Control of cell division in bacteria. Bacteriol Rev. 1974 Jun;38(2):199–221. doi: 10.1128/br.38.2.199-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


