Abstract
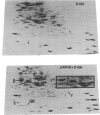
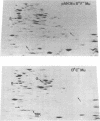
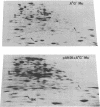
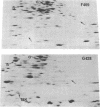
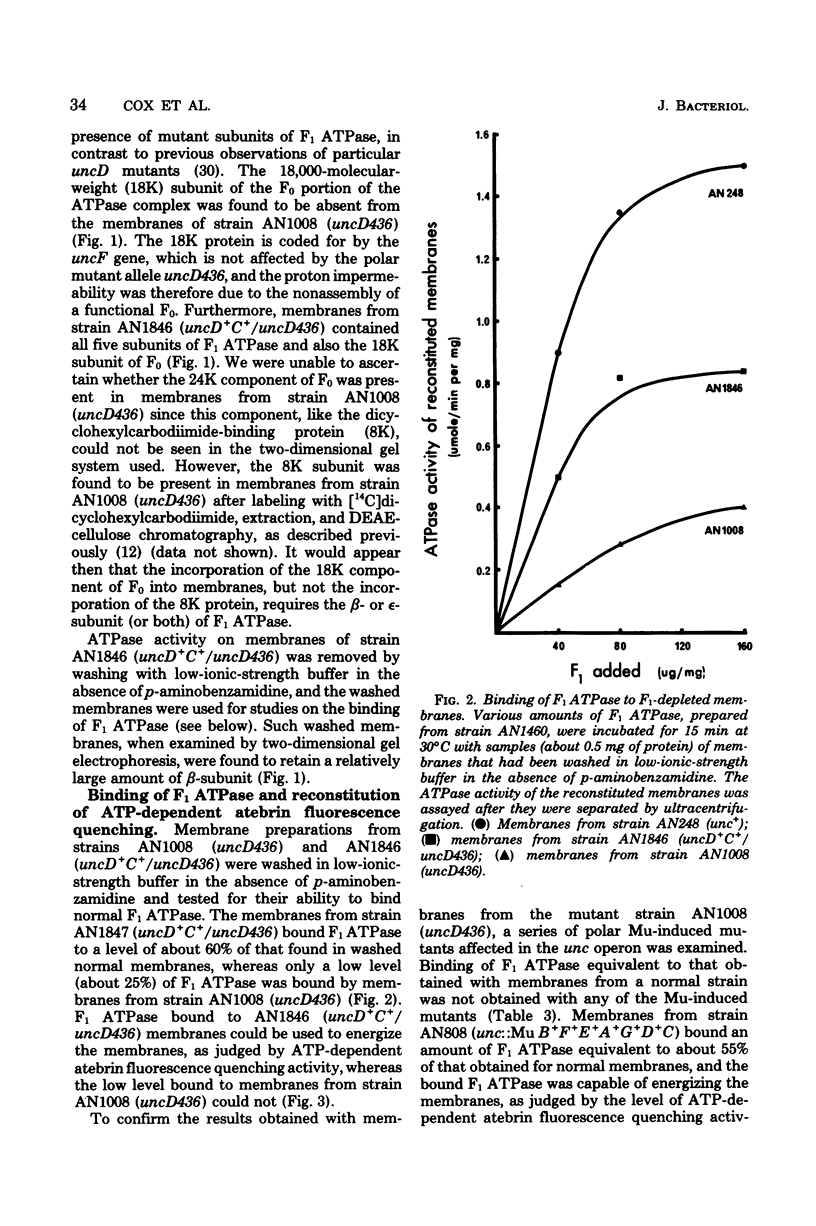
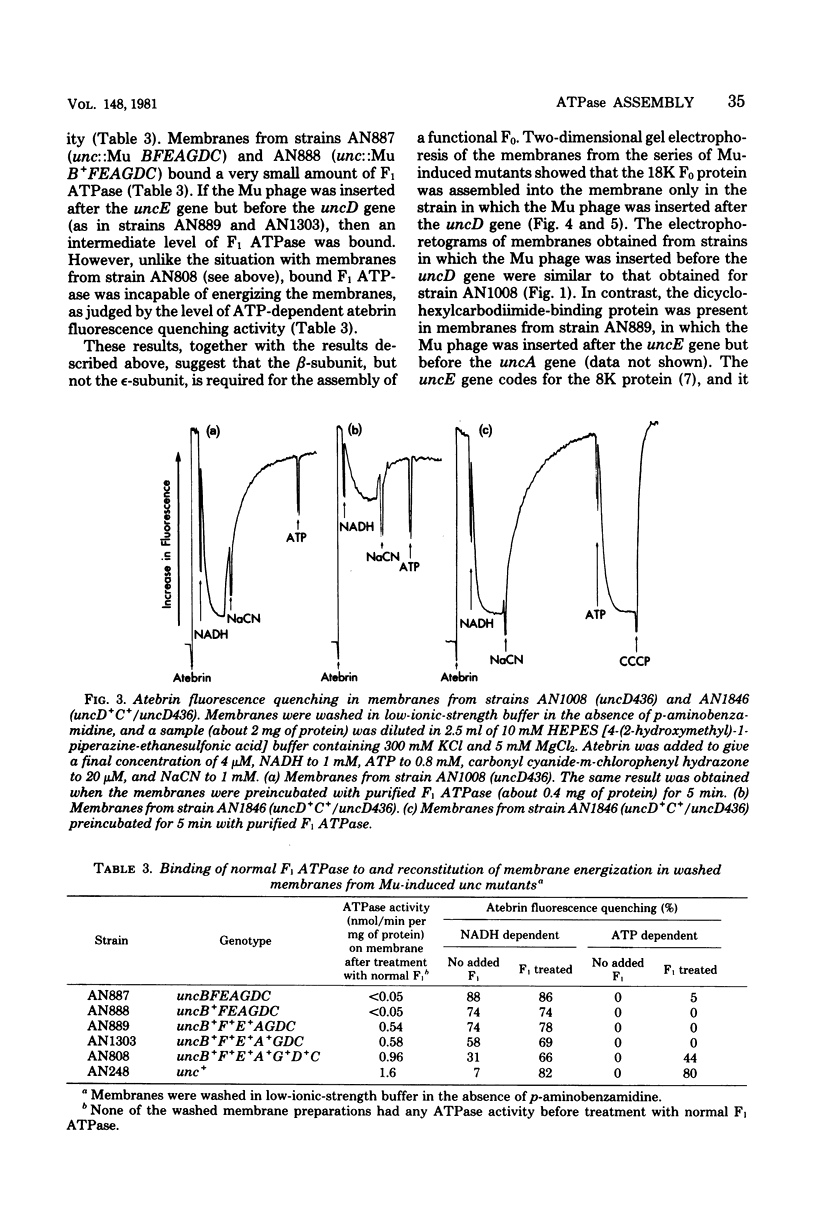
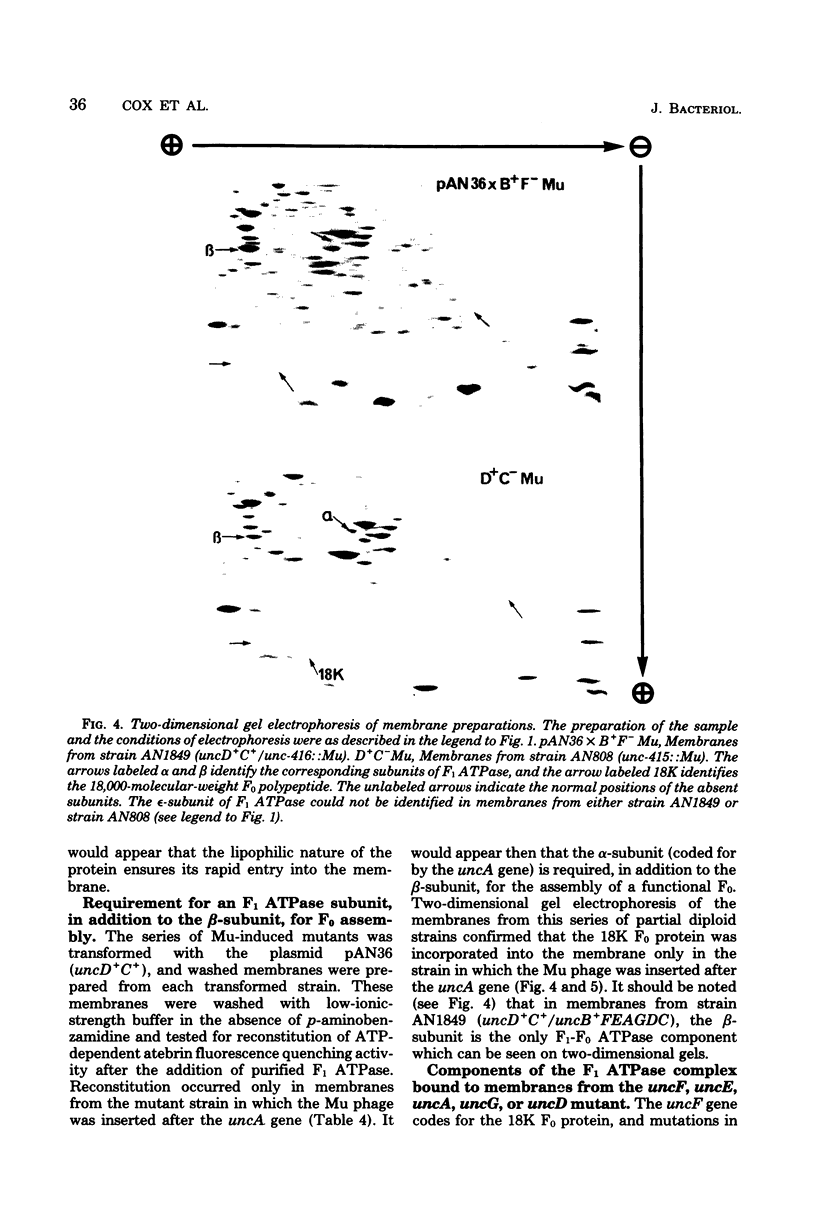
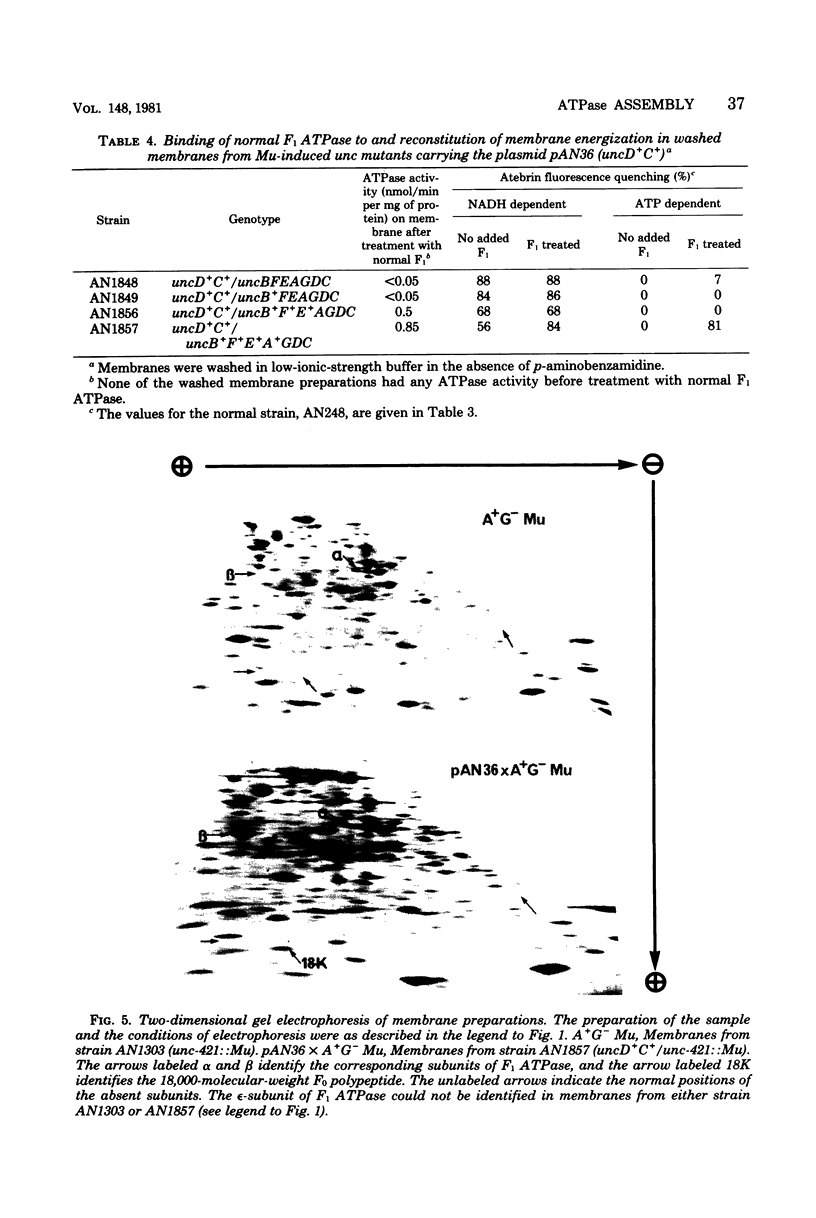
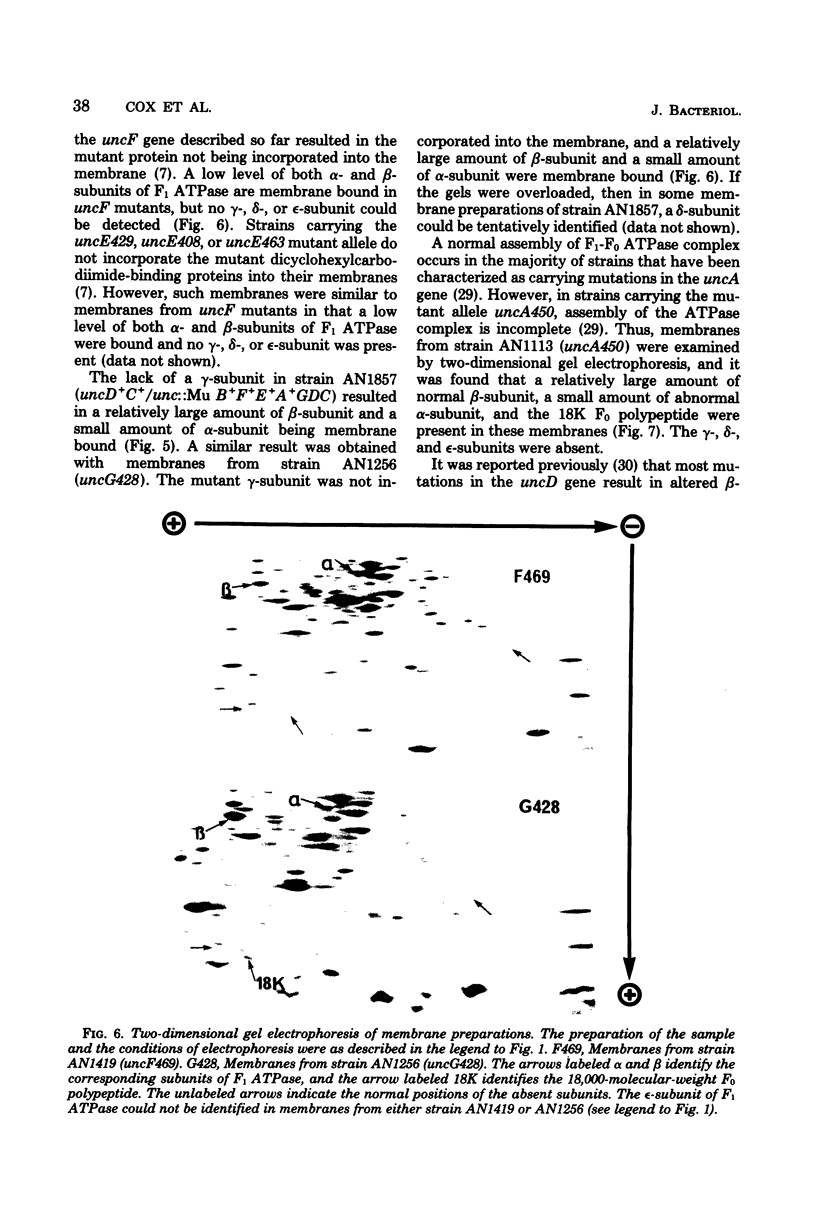
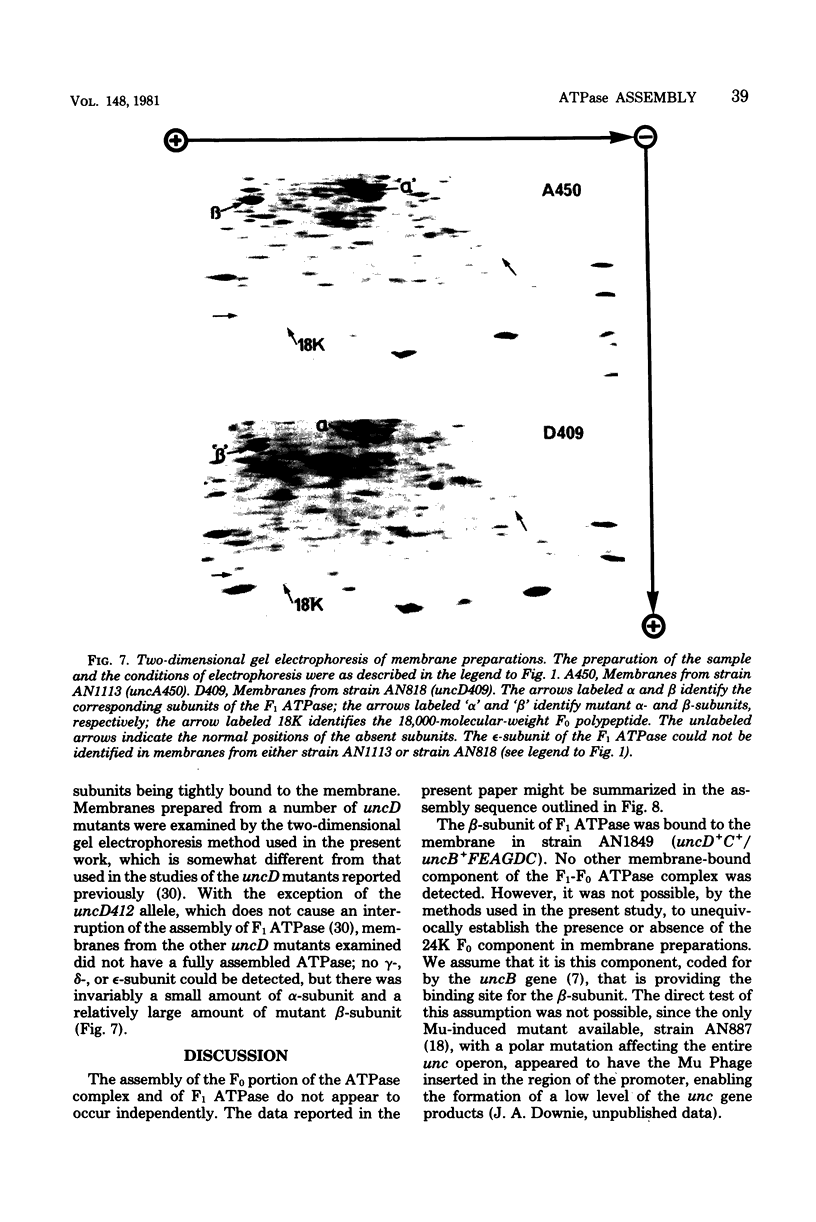
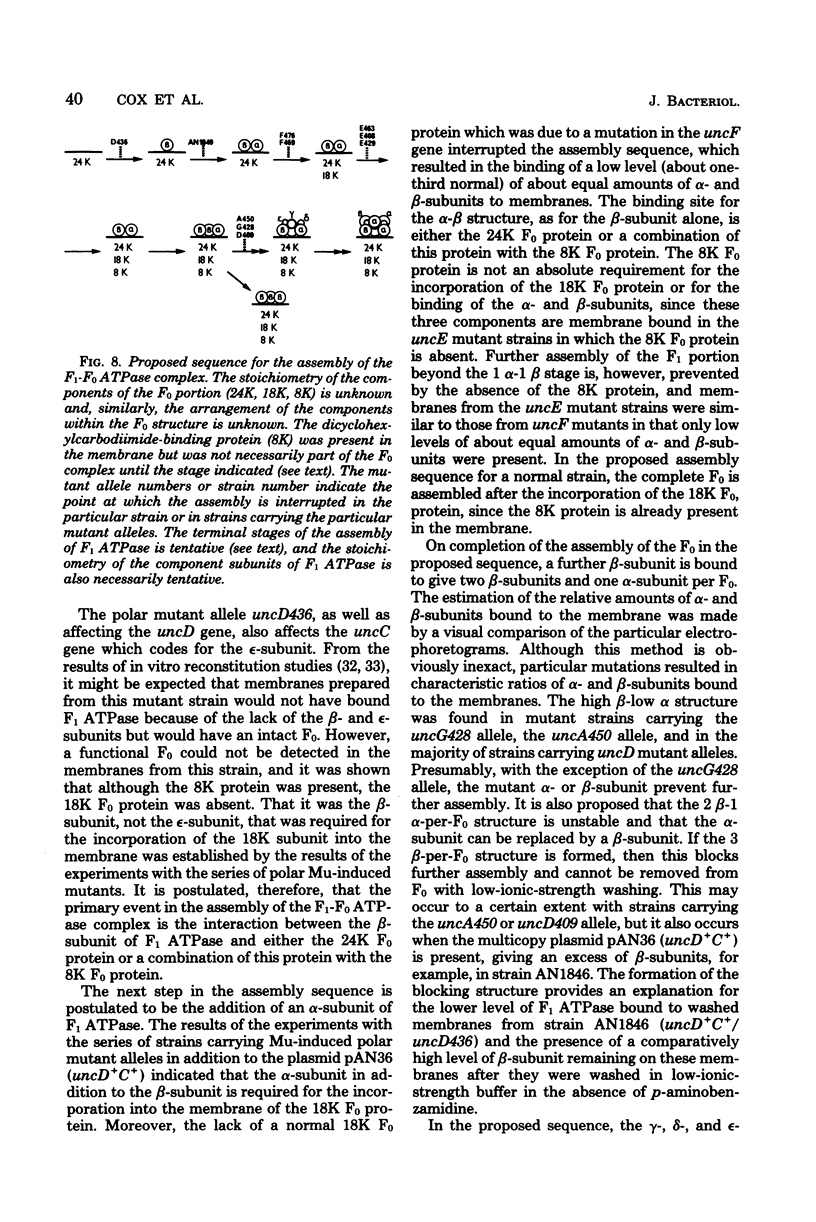
A strain of Escherichia coli (AN1007) carrying the polar uncD436 allele which affects the operon coding for the F1-F0 adenosine triphosphatase (ATPase) complex was isolated and characterized. The uncD436 allele affected the two genes most distal to the operon promoter, i.e., uncD and uncC. Although the genes coding for the F0 portion of the ATPase complex were not affected in strains carrying this mutant allele, the lack of reconstitution of washed membranes by normal F1 ATPase suggested that a functional F0 might not be formed. This conclusion was supported by the observation that the 18,000-molecular-weight F0 subunit, coded for by the uncF gene, was absent from the membranes. Plasmid pAN36 (uncD+C+), when inserted into a strain carrying the uncD436 allele, resulted in the incorporation of the 18,000-molecular-weight F0 subunit into the membrane. A further series of experiments with Mu-induced polarity mutants, with and without plasmid pAN36, showed that the formation of both the alpha- and beta-subunits of F1 ATPase was an essential prerequisite to the incorporation into the membrane of the 18,000-molecular-weight F0 subunit and to the formation of a functional F0. Examination of the polypeptide composition of membranes from various unc mutants allowed a sequence for the normal assembly of the F1-F0 ATPase complex to be proposed.
Full text
PDF


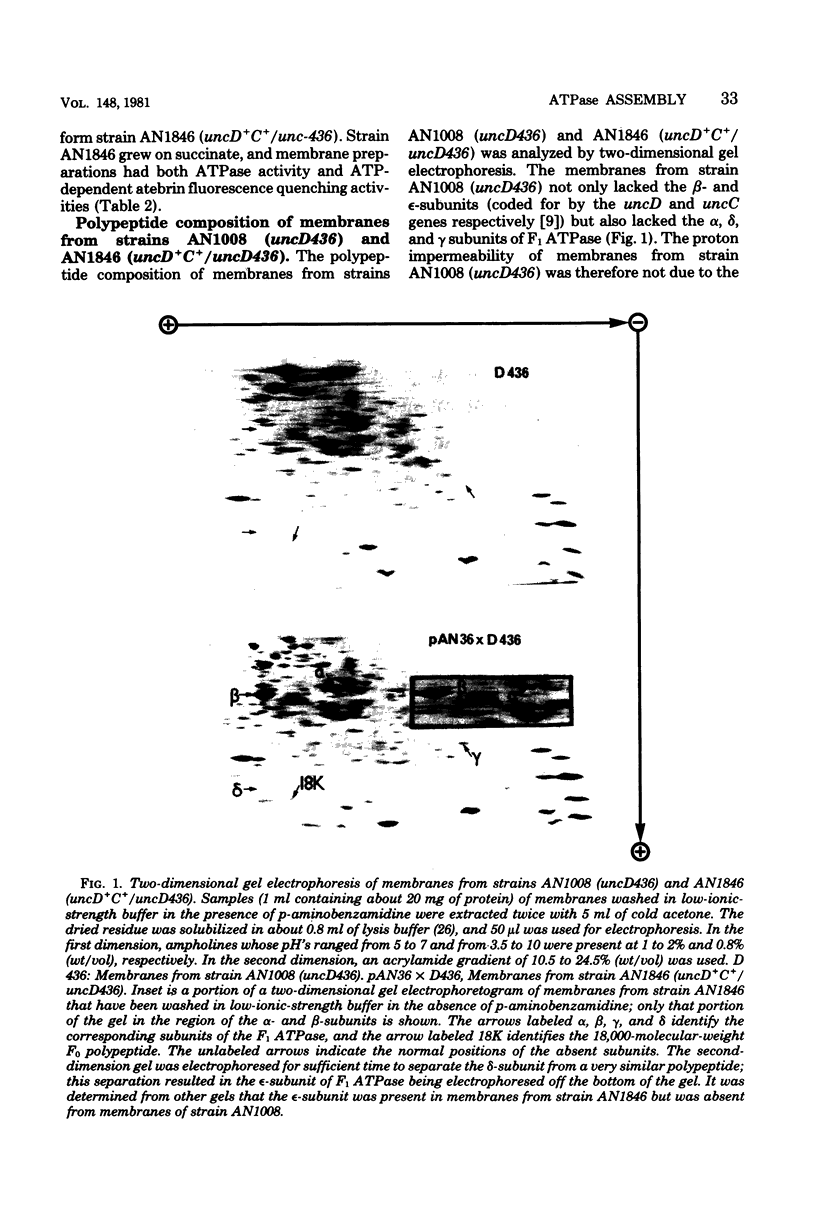


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. A cross-linking study of the Ca2+, Mg2+-activated adenosine triphosphatase of Escherichia coli. Eur J Biochem. 1980 May;106(2):495–503. doi: 10.1111/j.1432-1033.1980.tb04596.x. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- Cohen N. S., Lee S. H., Brodie A. F. Purification and characteristics of hydrophobic membrane protein(s) required for DCCD sensitivity of ATPase in Mycobacterium phlei. J Supramol Struct. 1978;8(1):111–117. doi: 10.1002/jss.400080109. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Downie J. A., Fayle D. R., Gibson F., Radik J. Inhibition, by a protease inhibitor, of the solubilization of the F1-portion of the Mg2+-stimulated adenosine triphosphatase of Escherichia coli. J Bacteriol. 1978 Jan;133(1):287–292. doi: 10.1128/jb.133.1.287-292.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Downie J. A., Gibson F., Radik J. Genetic complementation between two mutant unc alleles (unc A401 and unc D409) affecting the Fl portion of the magnesium ion-stimulated adenosine triphosphatase of Escherichia coli K12. Biochem J. 1978 Mar 15;170(3):593–598. doi: 10.1042/bj1700593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Cox G. B., Langman L., Ash G., Becker M., Gibson F. Three genes coding for subunits of the membrane sector (F0) of the Escherichia coli adenosine triphosphatase complex. J Bacteriol. 1981 Jan;145(1):200–210. doi: 10.1128/jb.145.1.200-210.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Gibson F., Cox G. B. Membrane adenosine triphosphatases of prokaryotic cells. Annu Rev Biochem. 1979;48:103–131. doi: 10.1146/annurev.bi.48.070179.000535. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Langman L., Cox G. B., Yanofsky C., Gibson F. Subunits of the adenosine triphosphatase complex translated in vitro from the Escherichia coli unc operon. J Bacteriol. 1980 Jul;143(1):8–17. doi: 10.1128/jb.143.1.8-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Senior A. E., Gibson F., Cox G. B. A fifth gene (uncE) in the operon concerned with oxidative phosphorylation in Escherichia coli. J Bacteriol. 1979 Feb;137(2):711–718. doi: 10.1128/jb.137.2.711-718.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. D., Futai M. Reconstitution of a functional coupling factor from the isolated subunits of Escherichia coli F1 ATPase. J Biol Chem. 1980 Jan 10;255(1):113–118. [PubMed] [Google Scholar]
- Fillingame R. H. Purification of the carbodiimide-reactive protein component of the ATP energy-transducing system of Escherichia coli. J Biol Chem. 1976 Nov 10;251(21):6630–6637. [PubMed] [Google Scholar]
- Foster D. L., Fillingame R. H. Energy-transducing H+-ATPase of Escherichia coli. Purification, reconstitution, and subunit composition. J Biol Chem. 1979 Sep 10;254(17):8230–8236. [PubMed] [Google Scholar]
- Friedl P., Friedl C., Schairer H. U. The ATP synthetase of Escherichia coli K12: purification of the enzyme and reconstitution of energy-transducing activities. Eur J Biochem. 1979 Oct;100(1):175–180. doi: 10.1111/j.1432-1033.1979.tb02046.x. [DOI] [PubMed] [Google Scholar]
- Futai M., Kanazawa H., Takeda K., Kagawa Y. Reconstitution of ATPase from the isolated subunits of coupling factor F1's of Escherichia coli and thermophilic bacterium PS3. Biochem Biophys Res Commun. 1980 Sep 16;96(1):227–234. doi: 10.1016/0006-291x(80)91204-8. [DOI] [PubMed] [Google Scholar]
- Gibson F., Cox G. B., Downie J. A., Radik J. A mutation affecting a second component of the F0 portion of the magnesium ion-stimulated adenosine triphosphatase of Escherichia coli K12. The uncC424 allele. Biochem J. 1977 Apr 15;164(1):193–198. doi: 10.1042/bj1640193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Cox G. B., Downie J. A., Radik J. Partial diploids of Escherichia coli carrying normal and mutant alleles affecting oxidative phosphorylation. Biochem J. 1977 Mar 15;162(3):665–670. doi: 10.1042/bj1620665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Downie J. A., Cox G. B., Radik J. Mu-induced polarity in the unc operon of Escherichia coli. J Bacteriol. 1978 Jun;134(3):728–736. doi: 10.1128/jb.134.3.728-736.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Downie J. A. The reconstitution of functional respiratory chains in membranes from electron-transport-deficient mutants of Escherichia coli as demonstrated by quenching of atebrin fluorescence. Biochem J. 1974 Sep;142(3):703–706. doi: 10.1042/bj1420703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra V. K., Lee S. H., Ritz C. J., Brodie A. F. Purification and properties of membrane-bound coupling factor-latent ATPase from Mycobacterium phlei. J Supramol Struct. 1975;3(3):231–241. doi: 10.1002/jss.400030305. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murgola E. J., Yanofsky C. Structural interactions between amino acid residues at positions 22 and 211 in the tryptophan synthetase alpha chain of Escherichia coli. J Bacteriol. 1974 Feb;117(2):444–448. doi: 10.1128/jb.117.2.444-448.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrin R. S., Foster D. L., Fillingame R. H. Energy-transducing H+-ATPase of Escherichia coli. Reconstitution of proton translocation activity of the intrinsic membrane sector. J Biol Chem. 1980 Jun 25;255(12):5643–5648. [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Sone N., Hirata H., Yoshida M., Kagawa Y. Purified proton conductor in proton translocating adenosine triphosphatase of a thermophilic bacterium. J Biol Chem. 1977 Sep 10;252(17):6125–6131. [PubMed] [Google Scholar]
- Senior A. E., Downie J. A., Cox G. B., Gibson F., Langman L., Fayle D. R. The uncA gene codes for the alpha-subunit of the adenosine triphosphatase of Escherichia coli. Electrophoretic analysis of uncA mutant strains. Biochem J. 1979 Apr 15;180(1):103–109. doi: 10.1042/bj1800103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E., Fayle D. R., Downie J. A., Gibson F., Cox G. B. Properties of membranes from mutant strains of Escherichia coli in which the beta-subunit of the adenosine triphosphatase is abnormal. Biochem J. 1979 Apr 15;180(1):111–118. doi: 10.1042/bj1800111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Kagawa Y. Purification and properties of a dicyclohexylcarbodiimide-sensitive adenosine triphosphatase from a thermophilic bacterium. J Biol Chem. 1975 Oct 10;250(19):7917–7923. [PubMed] [Google Scholar]
- Sternweis P. C., Smith J. B. Characterization of the purified membrane attachment (beta) subunit of the proton translocating adenosine triphosphatase from Escherichia coli. Biochemistry. 1977 Sep 6;16(18):4020–4025. doi: 10.1021/bi00637a013. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C. The epsilon subunit of Escherichia coli coupling factor 1 is required for its binding to the cytoplasmic membrane. J Biol Chem. 1978 May 10;253(9):3123–3128. [PubMed] [Google Scholar]
- Yoshida M., Okamoto H., Sone N., Hirata H., Kagawa Y. Reconstitution of thermostable ATPase capable of energy coupling from its purified subunits. Proc Natl Acad Sci U S A. 1977 Mar;74(3):936–940. doi: 10.1073/pnas.74.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Sone N., Hirata H., Kagawa Y. Reconstitution of adenosine triphosphatase of thermophilic bacterium from purified individual subunits. J Biol Chem. 1977 May 25;252(10):3480–3485. [PubMed] [Google Scholar]