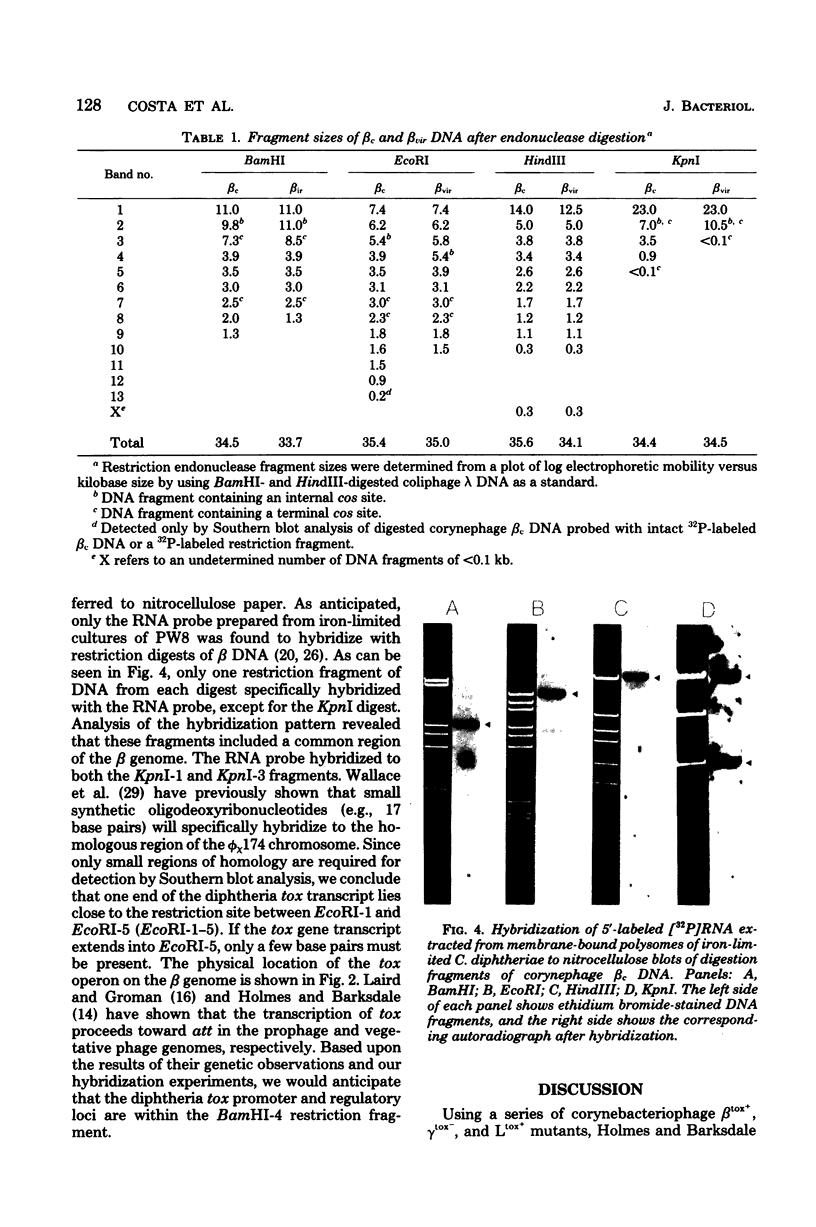
Abstract
The BamHI, EcoRI, HindIII, and KpnI restriction endonuclease maps of corynebacteriophage beta c and beta vir were constructed. beta vir appeared to be identical to beta c, except for an approximate 1-kilobase deletion that removed a BamHI site, two KpnI sites, and three EcoRI restriction sites. The diphtheria tox operon was located by hybridizing in vitro 32P-labeled tox messenger ribonucleic acid to blots of endonuclease-digested beta deoxyribonucleic acids. The messenger ribonucleic acid probe was found to hybridize to a 2.1-kilobase region of the beta genome. Since approximately 1.9 kilobases is required to encode prodiphtheria toxin, the data presented strongly suggest that the tox operon of beta is monocistronic.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARDSDALE W. L., PAPPENHEIMER A. M., Jr Phage-host relationships in nontoxigenic and toxigenic diphtheria bacilli. J Bacteriol. 1954 Feb;67(2):220–232. doi: 10.1128/jb.67.2.220-232.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck G. A., Groman N. B. Identification of deoxyribonucleic acid restriction fragments of beta-converting corynebacteriophages that carry the gene for diphtheria toxin. J Bacteriol. 1981 Oct;148(1):153–162. doi: 10.1128/jb.148.1.153-162.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck G. A., Groman N. B. Physical mapping of beta-converting and gamma-nonconverting corynebacteriophage genomes. J Bacteriol. 1981 Oct;148(1):131–142. doi: 10.1128/jb.148.1.131-142.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck G., Groman N., Falkow S. Relationship between beta converting and gamma non-converting corynebacteriophage DNA. Nature. 1978 Feb 16;271(5646):683–685. doi: 10.1038/271683a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 1979 Sep 11;7(1):179–192. doi: 10.1093/nar/7.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN V. J., MORSE I. U. Further observations on the change to virulence of bacteriophage-infected a virulent strains of Corynebacterium diphtheria. J Bacteriol. 1952 Mar;63(3):407–414. doi: 10.1128/jb.63.3.407-414.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN V. J. Studies on the virulence of bacteriophage-infected strains of Corynebacterium diphtheriae. J Bacteriol. 1951 Jun;61(6):675–688. doi: 10.1128/jb.61.6.675-688.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B., EATON M., BOOHER Z. K. Studies of mono- and polylysogenic Corynebacterium diphtheriae. J Bacteriol. 1958 Mar;75(3):320–325. doi: 10.1128/jb.75.3.320-325.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B., EATON M. Genetic factors in Corynebacterium diphtheriae conversion. J Bacteriol. 1955 Dec;70(6):637–640. doi: 10.1128/jb.70.6.637-640.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B. Evidence for the active role of bacteriophage in the conversion of nontoxigenic Corynebacterium diphtheriae to toxin production. J Bacteriol. 1955 Jan;69(1):9–15. doi: 10.1128/jb.69.1.9-15.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Barksdale L. Genetic analysis of tox+ and tox- bacteriophages of Corynebacterium diphtheriae. J Virol. 1969 Jun;3(6):586–598. doi: 10.1128/jvi.3.6.586-598.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W., Groman N. Orientation of the tox gene in the prophage of corynebacteriophage beta. J Virol. 1976 Jul;19(1):228–231. doi: 10.1128/jvi.19.1.228-231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W., Groman N. Prophage map of converting corynebacteriophage beta. J Virol. 1976 Jul;19(1):208–219. doi: 10.1128/jvi.19.1.208-219.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Kanei C., Yoneda M. Temperature-sensitive mutants on nonlysogenizing corynebacteriophage hv : their isolation, characterization and relation to toxiongenesis. Biken J. 1971 Jun;14(2):119–129. [PubMed] [Google Scholar]
- Miller P. A., Pappenheimer A. M., Jr, Doolittle W. F. Phage-host relationships in certain strains of Corynebacterium diphtheriae. Virology. 1966 Jul;29(3):410–425. doi: 10.1016/0042-6822(66)90217-0. [DOI] [PubMed] [Google Scholar]
- Murphy J. R., Michel J. L., Teng M. Evidence that the regulation of diphtheria toxin production is directed at the level of transcription. J Bacteriol. 1978 Aug;135(2):511–516. doi: 10.1128/jb.135.2.511-516.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Pappenheimer A. M., Jr, de Borms S. T. Synthesis of diphtheria tox-gene products in Escherichia coli extracts. Proc Natl Acad Sci U S A. 1974 Jan;71(1):11–15. doi: 10.1073/pnas.71.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Singer R. A. Temperature-sensitive mutants of toxinogenic corynebacteriophage beta. I. Genetics. Virology. 1973 Oct;55(2):347–356. doi: 10.1016/0042-6822(73)90174-8. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Murphy J. R., Davis B. D. Precursor in cotranslational secretion of diphtheria toxin. J Bacteriol. 1980 Jan;141(1):184–189. doi: 10.1128/jb.141.1.184-189.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Uchida T., Gill D. M., Pappenheimer A. M., Jr Mutation in the structural gene for diphtheria toxin carried by temperate phage . Nat New Biol. 1971 Sep 1;233(35):8–11. doi: 10.1038/newbio233008a0. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Zain B. S., Roberts R. J. Sequences from the beginning of the fiber messenger RNA of adenovirus-2. J Mol Biol. 1979 Jun 25;131(2):341–352. doi: 10.1016/0022-2836(79)90080-9. [DOI] [PubMed] [Google Scholar]