Abstract
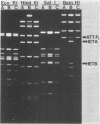
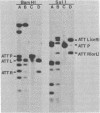
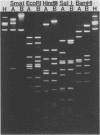
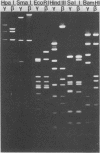
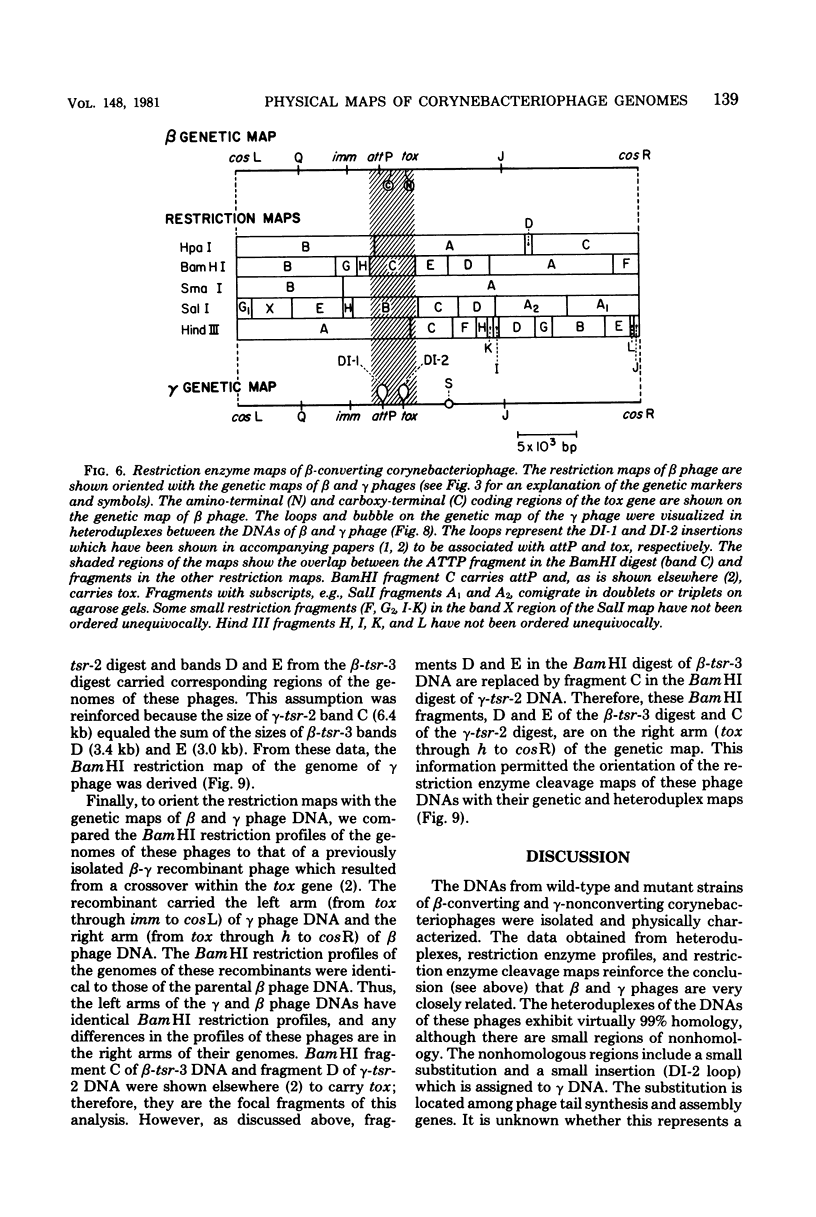
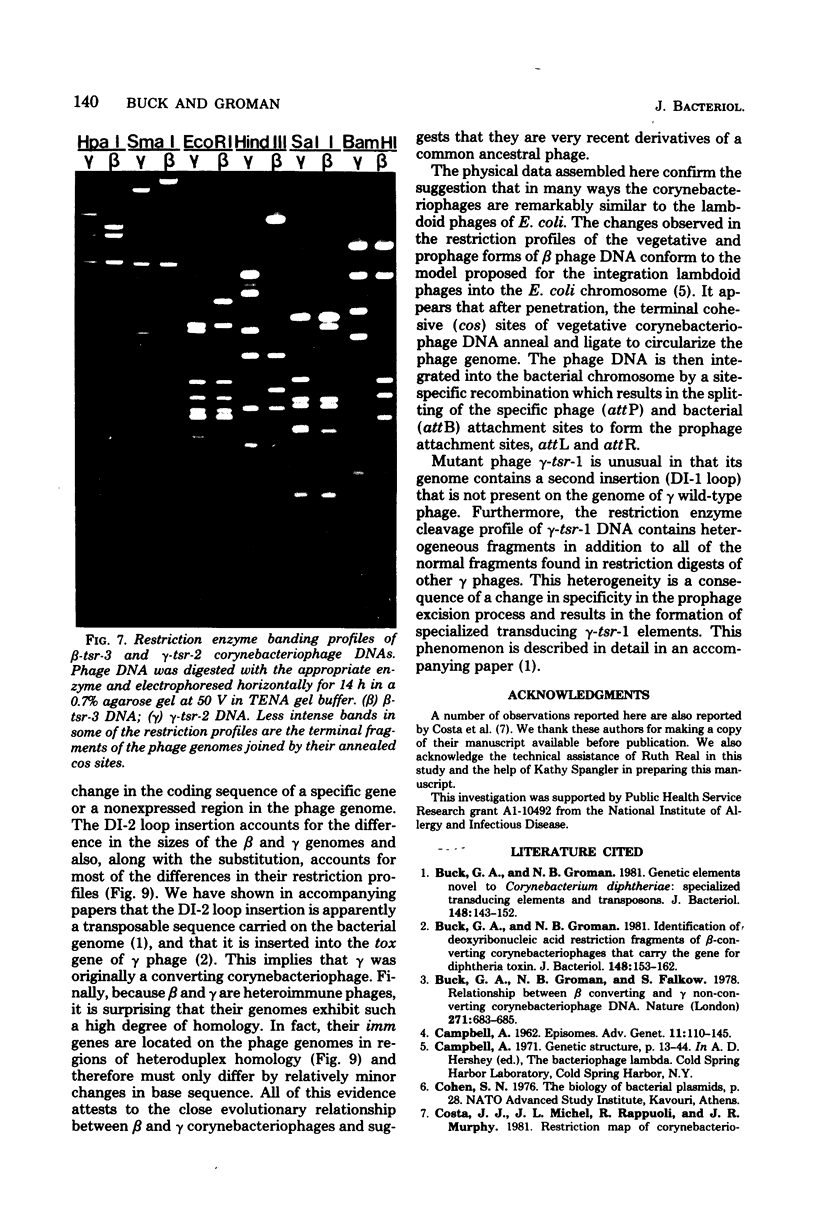
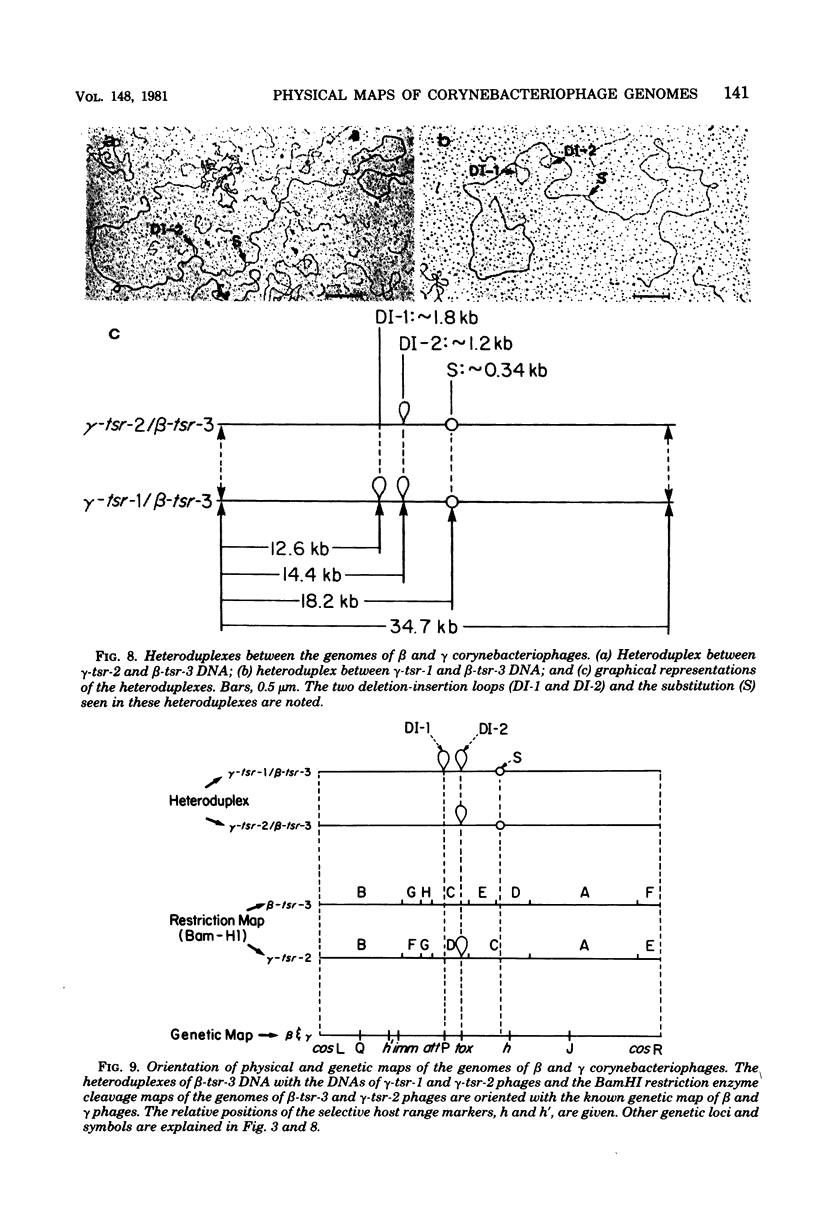
Deoxyribonucleic acids (DNAs) from wild-type and mutant strains of beta-converting and gamma-nonconverting corynebacteriophages were isolated and physically characterized. The data obtained from DNA heteroduplexes, restriction enzyme banding profiles, and restriction maps reinforce the conclusion that beta and gamma phages are very closely related. The major physical differences seen in the DNA heteroduplexes are a small substitution bubble and one or two insertions which are present on the gamma phage genome. The insertions account for the differences in the genome sizes of beta and gamma phages, and with the substitution they are responsible for most of the differences in the restriction endonuclease profiles and maps of the corynebactriophage genomes, two special sites and the DNA fragments carrying them were identified. These were the cohesive (cos) sites and the specific attachment (attP) site of the vegetative phage genome. The behavior of these sites indicated that the transition of phage DNA from the vegetative to the prophage state involves the circularization of vegetative DNA through the cos sites and its integration into the bacterial chromosome via the attP site. The mechanism of corynebacteriophage integration was similar to that employed by Escherichia coli phage gamma. From the data assembled the physical and genetic maps of beta and gamma phage were oriented with respect to one another. The extensive similarity in their maps provides additional confirmation of a close evolutionary relationship.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck G. A., Groman N. B. Genetic elements novel for Corynebacterium diphtheriae: specialized transducing elements and transposons. J Bacteriol. 1981 Oct;148(1):143–152. doi: 10.1128/jb.148.1.143-152.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck G. A., Groman N. B. Identification of deoxyribonucleic acid restriction fragments of beta-converting corynebacteriophages that carry the gene for diphtheria toxin. J Bacteriol. 1981 Oct;148(1):153–162. doi: 10.1128/jb.148.1.153-162.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck G., Groman N., Falkow S. Relationship between beta converting and gamma non-converting corynebacteriophage DNA. Nature. 1978 Feb 16;271(5646):683–685. doi: 10.1038/271683a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- FREEMAN V. J. Studies on the virulence of bacteriophage-infected strains of Corynebacterium diphtheriae. J Bacteriol. 1951 Jun;61(6):675–688. doi: 10.1128/jb.61.6.675-688.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B., EATON M., BOOHER Z. K. Studies of mono- and polylysogenic Corynebacterium diphtheriae. J Bacteriol. 1958 Mar;75(3):320–325. doi: 10.1128/jb.75.3.320-325.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B., EATON M. Genetic factors in Corynebacterium diphtheriae conversion. J Bacteriol. 1955 Dec;70(6):637–640. doi: 10.1128/jb.70.6.637-640.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman N. B., Rabin M. Superinfection exclusion by heteroimmune corynebacteriophages. J Virol. 1980 Nov;36(2):526–532. doi: 10.1128/jvi.36.2.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman N., Laird W. Heat-inducible mutants of corynebacteriophage. J Virol. 1977 Sep;23(3):587–591. doi: 10.1128/jvi.23.3.587-591.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Barksdale L. Comparative studies with tox plus and tox minus corynebacteriophages. J Virol. 1970 Jun;5(6):783–784. doi: 10.1128/jvi.5.6.783-794.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Barksdale L. Genetic analysis of tox+ and tox- bacteriophages of Corynebacterium diphtheriae. J Virol. 1969 Jun;3(6):586–598. doi: 10.1128/jvi.3.6.586-598.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K. Characterization and genetic mapping of nontoxinogenic (tox) mutants of corynebacteriophage beta. J Virol. 1976 Jul;19(1):195–207. doi: 10.1128/jvi.19.1.195-207.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W., Groman N. Orientation of the tox gene in the prophage of corynebacteriophage beta. J Virol. 1976 Jul;19(1):228–231. doi: 10.1128/jvi.19.1.228-231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W., Groman N. Prophage map of converting corynebacteriophage beta. J Virol. 1976 Jul;19(1):208–219. doi: 10.1128/jvi.19.1.208-219.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Murphy J. R., Pappenheimer A. M., Jr, de Borms S. T. Synthesis of diphtheria tox-gene products in Escherichia coli extracts. Proc Natl Acad Sci U S A. 1974 Jan;71(1):11–15. doi: 10.1073/pnas.71.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M. W., Florini J. R. A rapid method for desalting small volumes of solution. Anal Biochem. 1973 Sep;55(1):328–330. doi: 10.1016/0003-2697(73)90325-4. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J., Groman N., Coyle M. Plasmids in Corynebacterium diphtheriae and diphtheroids mediating erythromycin resistance. Antimicrob Agents Chemother. 1980 Nov;18(5):814–821. doi: 10.1128/aac.18.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. A. Temperature-sensitive mutants of toxinogenic corynebacteriophage beta. I. Genetics. Virology. 1973 Oct;55(2):347–356. doi: 10.1016/0042-6822(73)90174-8. [DOI] [PubMed] [Google Scholar]
- Singer R. A. Temperature-sensitive mutants of toxinogenic corynebacteriophage beta. II. Properties of mutant phages. Virology. 1973 Oct;55(2):357–362. doi: 10.1016/0042-6822(73)90175-x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Flavell R. A. A method for the recovery of DNA from agarose gels. Nucleic Acids Res. 1978 Jul;5(7):2321–2332. doi: 10.1093/nar/5.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Greany R. Diphtheria toxin and related proteins. I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J Biol Chem. 1973 Jun 10;248(11):3838–3844. [PubMed] [Google Scholar]
- Villems R., Duggleby C. J., Broda P. Restriction endonuclease mapping of DNA using in situ digestion in two-dimensional gels. FEBS Lett. 1978 May 15;89(2):267–270. doi: 10.1016/0014-5793(78)80233-6. [DOI] [PubMed] [Google Scholar]