Abstract
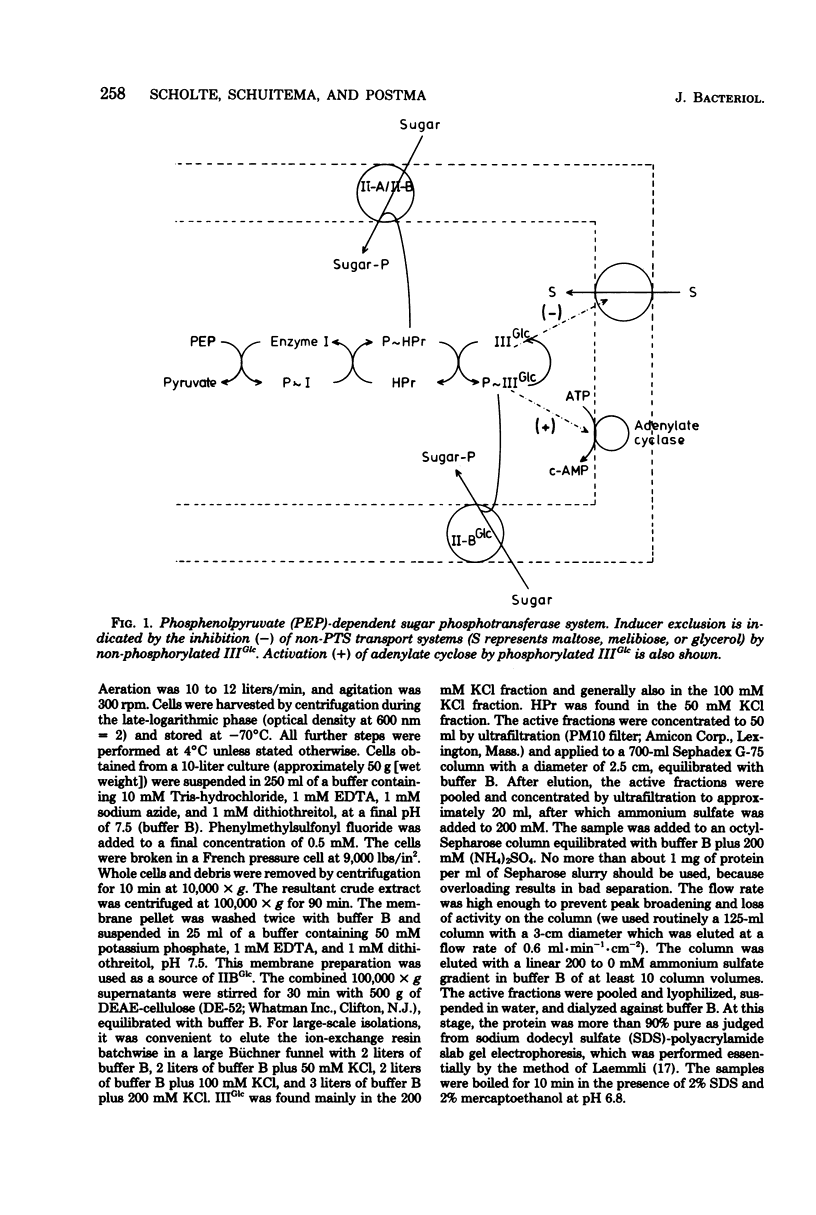
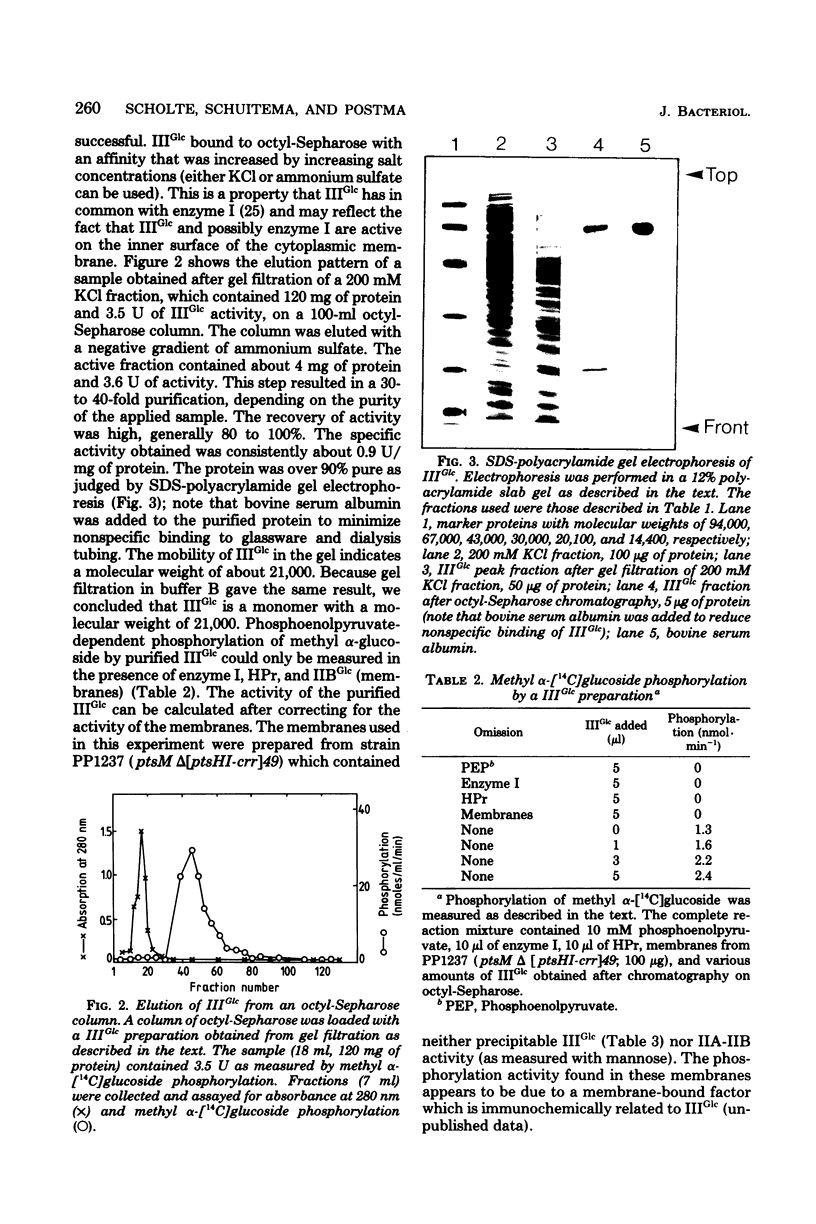
We report a procedure for the isolation of IIIglc of Salmonella typhimurium, a protein component of the phosphoenolpyruvate-dependent sugar phosphotransferase system. IIIGlc is a soluble protein with a molecular weight of 21,000, as determined by gel filtration and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The purified protein is involved in the phosphoenolpyruvate-dependent phosphorylation of methyl alpha-glucoside in vitro. Its affinity for octyl-Sepharose may be an indication of the partial hydrophobic nature of IIIGlc. A specific antiserum against purified IIIGlc was prepared. Growth on different carbon sources did not affect the synthesis of IIIGlc, as determined by quantitative immunoelectrophoresis. Mutations which lower the adenosine 3',5'-phosphate level, such as cya and pts, do not alter the IIIGlc level. The closely related enteric bacteria Escherichia coli and Klebsiella aerogenes contain a protein factor which is closely related to IIIGlc of S. typhimurium, whereas Staphylococcus aureus does not.
Full text
PDF

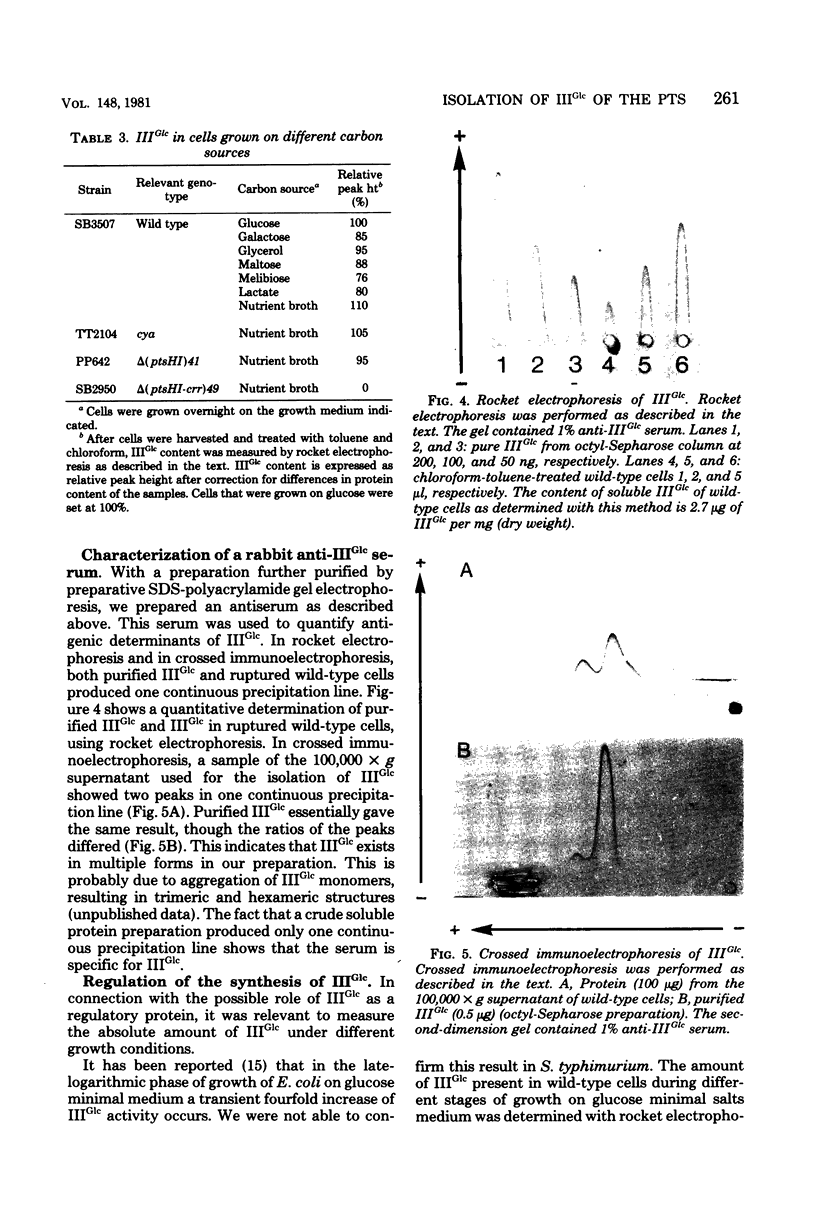
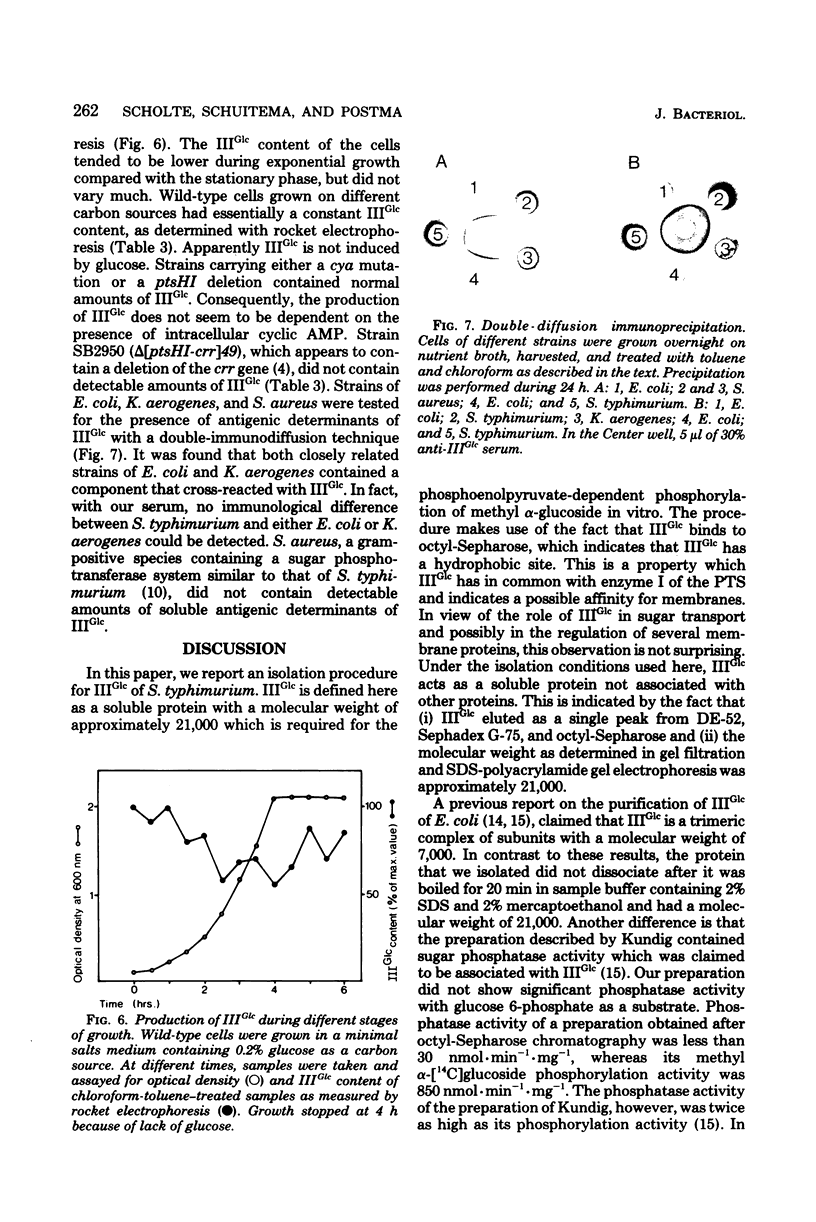


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolshakova T. N., Gabrielyan T. R., Bourd G. I., Gershanovitch V. N. Involvement of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system in regulation of transcription of catabolic genes. Eur J Biochem. 1978 Sep 1;89(2):483–490. doi: 10.1111/j.1432-1033.1978.tb12552.x. [DOI] [PubMed] [Google Scholar]
- Boos W., Lengeler J., Hermann K. O., Unsöld H. J. The regulation of the beta-methylgalactoside transport system and of the galactose binding protein of Escherichia coli K12. Eur J Biochem. 1971 Apr 30;19(4):457–470. doi: 10.1111/j.1432-1033.1971.tb01336.x. [DOI] [PubMed] [Google Scholar]
- Castro L., Feucht B. U., Morse M. L., Saier M. H., Jr Regulation of carbohydrate permeases and adenylate cyclase in Escherichia coli. Studies with mutant strains in which enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system is thermolabile. J Biol Chem. 1976 Sep 25;251(18):5522–5527. [PubMed] [Google Scholar]
- Cordaro J. C., Roseman S. Deletion mapping of the genes coding for HPr and enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):17–29. doi: 10.1128/jb.112.1.17-29.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi-Rontani C., Danchin A., Ullmann A. Catabolite repression in Escherichia coli mutants lacking cyclic AMP receptor protein. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5799–5801. doi: 10.1073/pnas.77.10.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. P., Gazdar C., Prasad C., Peterkofsky A., Curtis S. J., Epstein W. Involvement of the glucose enzymes II of the sugar phosphotransferase system in the regulation of adenylate cyclase by glucose in Escherichia coli. J Biol Chem. 1976 Apr 25;251(8):2462–2468. [PubMed] [Google Scholar]
- Harwood J. P., Peterkofsky A. Glucose-sensitive adenylate cyclase in toluene-treated cells of Escherichia coli B. J Biol Chem. 1975 Jun 25;250(12):4656–4662. [PubMed] [Google Scholar]
- Hengstenberg W. Enzymology of carbohydrate transport in bacteria. Curr Top Microbiol Immunol. 1977;77:97–126. doi: 10.1007/978-3-642-66740-4_4. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Kornberg H. L., Maltby R., Watts P. D. Role of the crr-gene in glucose uptake by Escherichia coli. FEBS Lett. 1977 Feb 15;74(1):17–19. doi: 10.1016/0014-5793(77)80742-4. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Watts P. D. tgs and crr: Genes involved in catabolite inhibition and inducer exclusion in Escherichia coli. FEBS Lett. 1979 Aug 15;104(2):313–316. doi: 10.1016/0014-5793(79)80841-8. [DOI] [PubMed] [Google Scholar]
- Kundig W. Molecular interactions in the bacterial phosphoenolpyruvate-phosphotransferase system (PTS). J Supramol Struct. 1974;2(5-6):695–814. doi: 10.1002/jss.400020514. [DOI] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Glucose inhibition of adenylate cyclase in intact cells of Escherichia coli B. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2324–2328. doi: 10.1073/pnas.71.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Coordinate regulation of adenylate cyclase and carbohydrate permeases by the phosphoenolpyruvate:sugar phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1975 Sep 10;250(17):7078–7080. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Regulation of carbohydrate transport activities in Salmonella typhimurium: use of the phosphoglycerate transport system to energize solute uptake. J Bacteriol. 1980 Feb;141(2):611–617. doi: 10.1128/jb.141.2.611-617.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. 2nducer exclusion and regulation of the melibiose, maltose, glycerol, and lactose transport systems by the phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6606–6615. [PubMed] [Google Scholar]
- Saier M. H., Jr, Straud H., Massman L. S., Judice J. J., Newman M. J., Feucht B. U. Permease-specific mutations in Salmonella typhimurium and Escherichia coli that release the glycerol, maltose, melibiose, and lactose transport systems from regulation by the phosphoenolpyruvate:sugar phosphotransferase system. J Bacteriol. 1978 Mar;133(3):1358–1367. doi: 10.1128/jb.133.3.1358-1367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte B. J., Postma P. W. Competition between two pathways for sugar uptake by the phosphoenolpyruvate-dependent sugar phosphotransferase system in Salmonella typhimurium. Eur J Biochem. 1981;114(1):51–58. doi: 10.1111/j.1432-1033.1981.tb06171.x. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Meadow N. D., Roseman S. Modified assay procedures for the phosphotransferase system in enteric bacteria. Anal Biochem. 1979 May;95(1):293–304. doi: 10.1016/0003-2697(79)90219-7. [DOI] [PubMed] [Google Scholar]
- Yang J. K., Bloom R. W., Epstein W. Catabolite and transient repression in Escherichia coli do not require enzyme I of the phosphotransferase system. J Bacteriol. 1979 Apr;138(1):275–279. doi: 10.1128/jb.138.1.275-279.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]