Abstract
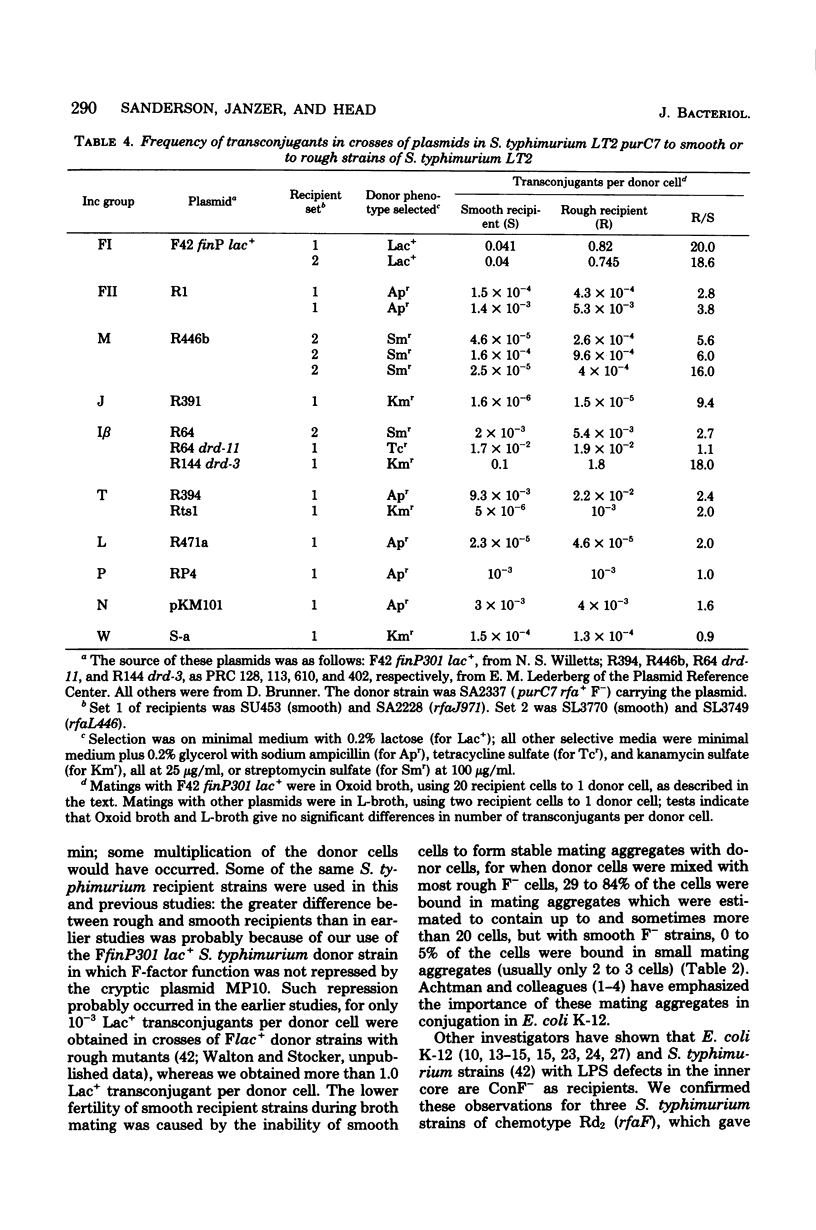
In crosses of Salmonella typhimurium FfinP301 lac+ to F- strains of S. typhimurium in broth, recipient strains which were rough mutants affected in the outer core region of the lipopolysaccharide gave an average of 1.4 Lac+ transconjugants per donor cell and over 50% of the donor and recipient cells in mating aggregates, whereas smooth recipient strains gave 0.08 Lac+ transconjugants and few cells in mating aggregates. Strains with mutations affecting the inner core of the lipopolysaccharide were usually poor recipients. When cells were mated on Millipore membrane filters, both smooth and rough strains gave ca. 1.0 Lac+ transconjugants per donor cell. Plasmids in Inc groups FI, FII, M, J, and I beta gave more transconjugants with rough than smooth strains, but there were no difference in crosses with plasmids in Inc groups T, L, P, N, and W. Strains with mutations in the ompA gene (deficient in Omp Ap = 33K = II* = conjugation protein) yielded only 0.02 Lac+ transconjugants per donor cell and few cells in mating aggregates. There was no indication of a deficiency of Omp Ap in smooth strains compared with rough strains. Reduced fertility of smooth recipients may occur because the O side chains of the lipopolysaccharide shield the recipient and reduce the frequency of stabilization of mating aggregates. However, gradient-of-transmission experiments indicated that once these mating aggregates are stabilized, they are equally stable in both smooth and rough recipients. Fertility was high in crosses of S. typhimurium Flac+ to Escherichia coli K-12 F- (0.75 Lac+ transconjugants per donor cell; over 50% of the cells in mating aggregates). In crosses of E. coli K-12 Flac+ to S. typhimurium smooth F-, ca. 10(-5) Lac+ transconjugants per donor cell were obtained; in crosses to rough recipient strains, fertility was increased 14-fold, and when the recipient was defective in the SA and LT host restriction systems, fertility was increased in additional 100-fold. Thus, both the lipopolysaccharide and the protein in the cell envelope of S. typhimurium were shown to be important in the recipient function in F-mediated conjugation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M. Mating aggregates in Escherichia coli conjugation. J Bacteriol. 1975 Aug;123(2):505–515. doi: 10.1128/jb.123.2.505-515.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Morelli G., Schwuchow S. Cell-cell interactions in conjugating Escherichia coli: role of F pili and fate of mating aggregates. J Bacteriol. 1978 Sep;135(3):1053–1061. doi: 10.1128/jb.135.3.1053-1061.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boro H., Brenchley J. E. A new generalized transducing phage for Salmonella typhimurium LT2. Virology. 1971 Sep;45(3):835–836. doi: 10.1016/0042-6822(71)90208-x. [DOI] [PubMed] [Google Scholar]
- Colson A. M., Colson C., Van Pel A. Host-controlled restriction mutants of Salmonella typhimurium. J Gen Microbiol. 1969 Sep;58(1):57–64. doi: 10.1099/00221287-58-1-57. [DOI] [PubMed] [Google Scholar]
- Colson C., Van Pel A. DNA restriction and modification systems in Salmonella. I. SA and SB, two Salmonella typhimurium systems determined by genes with a chromosomal location comparable to that of the Escherichia coli hsd genes. Mol Gen Genet. 1974 Apr 3;129(4):325–337. doi: 10.1007/BF00265696. [DOI] [PubMed] [Google Scholar]
- Dunny G. M., Brown B. L., Clewell D. B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham J. O., 3rd, Curtiss R., 3rd Isolation and characterization of conjugation-deficient mutants of Escherichia coli K-12. J Bacteriol. 1976 Jun;126(3):1194–1206. doi: 10.1128/jb.126.3.1194-1206.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan D. J., Willetts N. S. Two classes of Flac mutants insensitive to transfer inhibition by an F-like R factor. Mol Gen Genet. 1971;111(3):256–264. doi: 10.1007/BF00433110. [DOI] [PubMed] [Google Scholar]
- Gasson M. J., Willetts N. S. Five control systems preventing transfer of Escherichia coli K-12 sex factor F. J Bacteriol. 1975 May;122(2):518–525. doi: 10.1128/jb.122.2.518-525.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes L. M., Hoekstra W. P. Characterization of an Escherichia coli K-12 F-Con-mutant. J Bacteriol. 1976 May;126(2):593–600. doi: 10.1128/jb.126.2.593-600.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes L. M., Lugtenberg B. J., Hoekstra W. P. Conjugation deficient E. coli K12 F- mutants with heptose-less lipopolysaccharide. Mol Gen Genet. 1976 Jul 5;146(1):43–50. doi: 10.1007/BF00267981. [DOI] [PubMed] [Google Scholar]
- Havekes L., Tommassen J., Hoekstra W., Lugtenberg B. Isolation and characterization of Escherichia coli K-12 F- mutants defective in conjugation with an I-type donor. J Bacteriol. 1977 Jan;129(1):1–8. doi: 10.1128/jb.129.1.1-8.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., Chatterjee A. K., Sanderson K. E., Costerton J. W. Comparison of the cell envelope structure of a lipopolysaccharide-defective (heptose-deficient) strain and a smooth strain of Salmonella typhimurium. J Bacteriol. 1975 Nov;124(2):930–941. doi: 10.1128/jb.124.2.930-941.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T. T., Stocker B. A. ES18, a general transducing phage for smooth and nonsmooth Salmonella typhimurium. Virology. 1970 Nov;42(3):621–632. doi: 10.1016/0042-6822(70)90308-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Hellerqvist C. G. Rough mutants of Salmonella typhimurium: immunochemical and structural analysis of lipopolysaccharides from rfaH mutants. J Gen Microbiol. 1980 Jan;116(1):25–32. doi: 10.1099/00221287-116-1-25. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Reeves P. Recipient ability of bacteriophage-resistant mutants of Escherichia coli K-12. J Bacteriol. 1975 Oct;124(1):576–577. doi: 10.1128/jb.124.1.576-577.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monner D. A., Jonsson S., Boman H. G. Ampicillin-resistant mutants of Escherichia coli K-12 with lipopolysaccharide alterations affecting mating ability and susceptibility to sex-specific bacteriophages. J Bacteriol. 1971 Aug;107(2):420–432. doi: 10.1128/jb.107.2.420-432.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Ou J. T., Reim R. Effect of 1,10-phenanthroline on bacterial conjugation in Escherichia coli K-12: inhibition of maturation from preliminary mates into effective mates. J Bacteriol. 1976 Oct;128(1):363–371. doi: 10.1128/jb.128.1.363-371.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M. Escherichia coli females defective in conjugation and in adsorption of a single-stranded deoxyribonucleic acid phage. J Bacteriol. 1974 Jul;119(1):183–191. doi: 10.1128/jb.119.1.183-191.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roantree R. J., Kuo T. T., MacPhee D. G. The effect of defined lipopolysaccharide core defects upon antibiotic resistances of Salmonella typhimurium. J Gen Microbiol. 1977 Dec;103(2):223–234. doi: 10.1099/00221287-103-2-223. [DOI] [PubMed] [Google Scholar]
- SANDERSON K. E., DEMEREC M. THE LINKAGE MAP OF SALMONELLA TYPHIMURIUM. Genetics. 1965 Jun;51:897–913. doi: 10.1093/genetics/51.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Henning U. Action of a major outer cell envelope membrane protein in conjugation of Escherichia coli K-12. J Bacteriol. 1977 Mar;129(3):1651–1652. doi: 10.1128/jb.129.3.1651-1652.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Hancock R. E., Reeves P. Con--mutants: class of mutants in Escherichia coli K-12 lacking a major cell wall protein and defective in conjugation and adsorption of a bacteriophage. J Bacteriol. 1974 Sep;119(3):726–735. doi: 10.1128/jb.119.3.726-735.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. R., Humphreys G. O., Grindley N. D., Grindley J. N., Anderson E. S. Molecular studies of an fi+ plasmid from strains of Salmonella typhimurium. Mol Gen Genet. 1973 Nov 2;126(2):143–151. doi: 10.1007/BF00330989. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Rowbury R. J. The plasmid of Salmonella typhimurium LT2. Mol Gen Genet. 1973 Mar 19;121(4):347–353. doi: 10.1007/BF00433233. [DOI] [PubMed] [Google Scholar]
- Stocker B. A., Males B. M., Takano W. Salmonella typhimurium mutants of RfaH-phenotype: genetics and antibiotic sensitivities. J Gen Microbiol. 1980 Jan;116(1):17–24. doi: 10.1099/00221287-116-1-17. [DOI] [PubMed] [Google Scholar]
- Stocker B. A., Nurminen M., Mäkelä P. H. Mutants defective in the 33K outer membrane protein of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):376–383. doi: 10.1128/jb.139.2.376-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alphen L., Havekes L., Lugtenberg B. Major outer membrane protein d of Escherichia coli K12. Purification and in vitro activity of bacteriophages k3 and f-pilus mediated conjugation. FEBS Lett. 1977 Mar 15;75(1):285–290. doi: 10.1016/0014-5793(77)80104-x. [DOI] [PubMed] [Google Scholar]
- Van Pel A., Colson C. DNA restriction and modification systems in Salmonella. II. Genetic complementation between the K and B systems of Escherichia coli and the Salmonella typhimurium system SB, with the same chromosomal location. Mol Gen Genet. 1974;135(1):51–60. doi: 10.1007/BF00433901. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Arai T., Hattori T. Effects of cell wall polysaccharide on the mating ability of Salmonella typhimurium. Nature. 1970 Jan 3;225(5227):70–71. doi: 10.1038/225070a0. [DOI] [PubMed] [Google Scholar]
- Wiedemann B., Schmidt G. Die Bedeutung der Lipopolysaccharid-Struktur von E. coli für die Ubertragung von R-Faktoren. Zentralbl Bakteriol Orig A. 1972 Feb;219(2):180–186. [PubMed] [Google Scholar]
- Wiedemann B., Schmidt G. The problems of drug-resistant pathogenic bacteria. Experimental and clinical aspects of resistance determinants. Structure and recipient ability in E. Coli mutants. Ann N Y Acad Sci. 1971 Jun 11;182:123–125. doi: 10.1111/j.1749-6632.1971.tb30651.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]