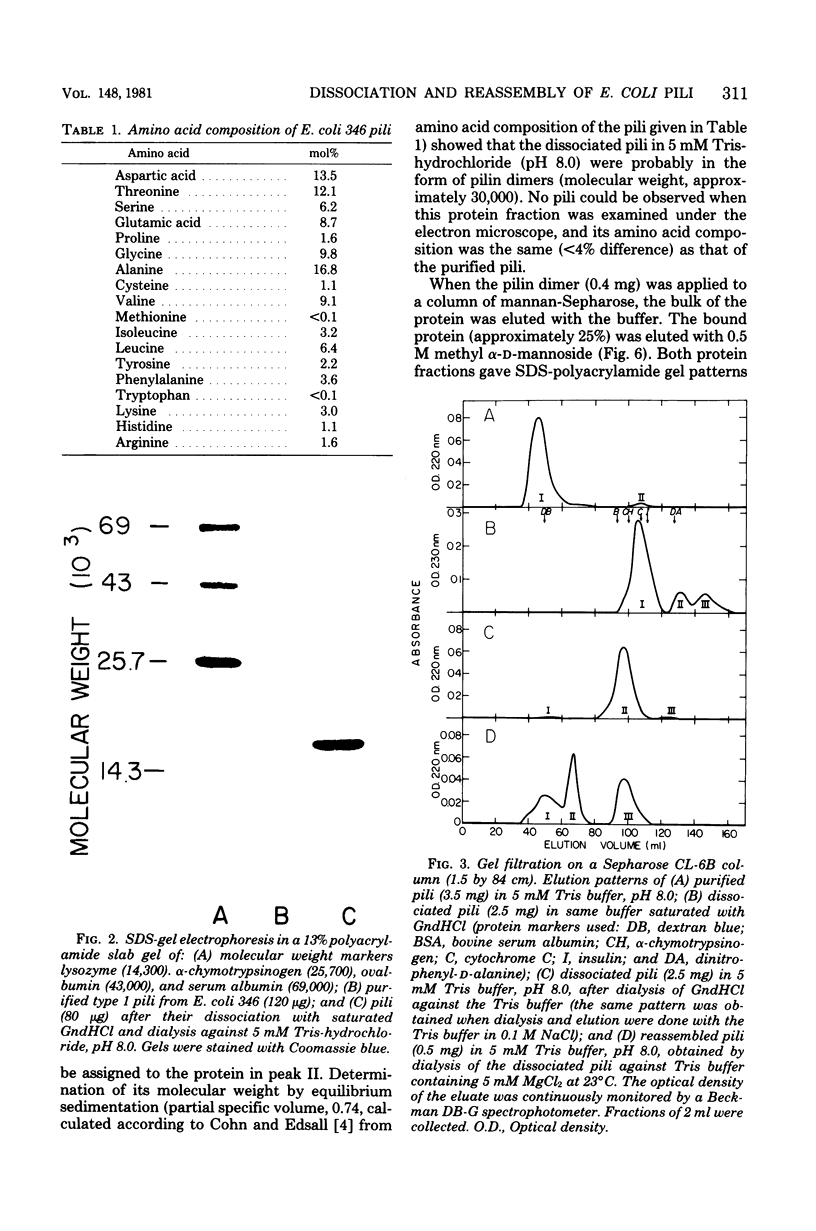
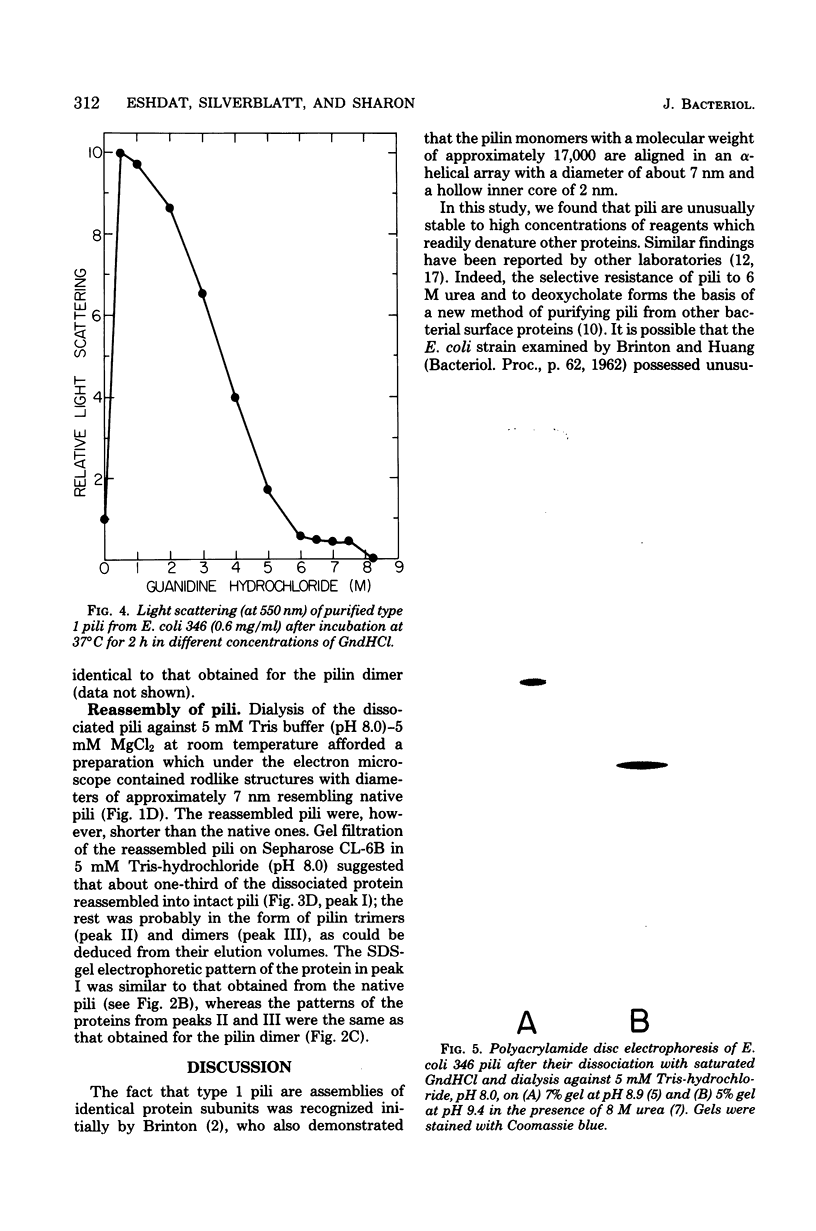
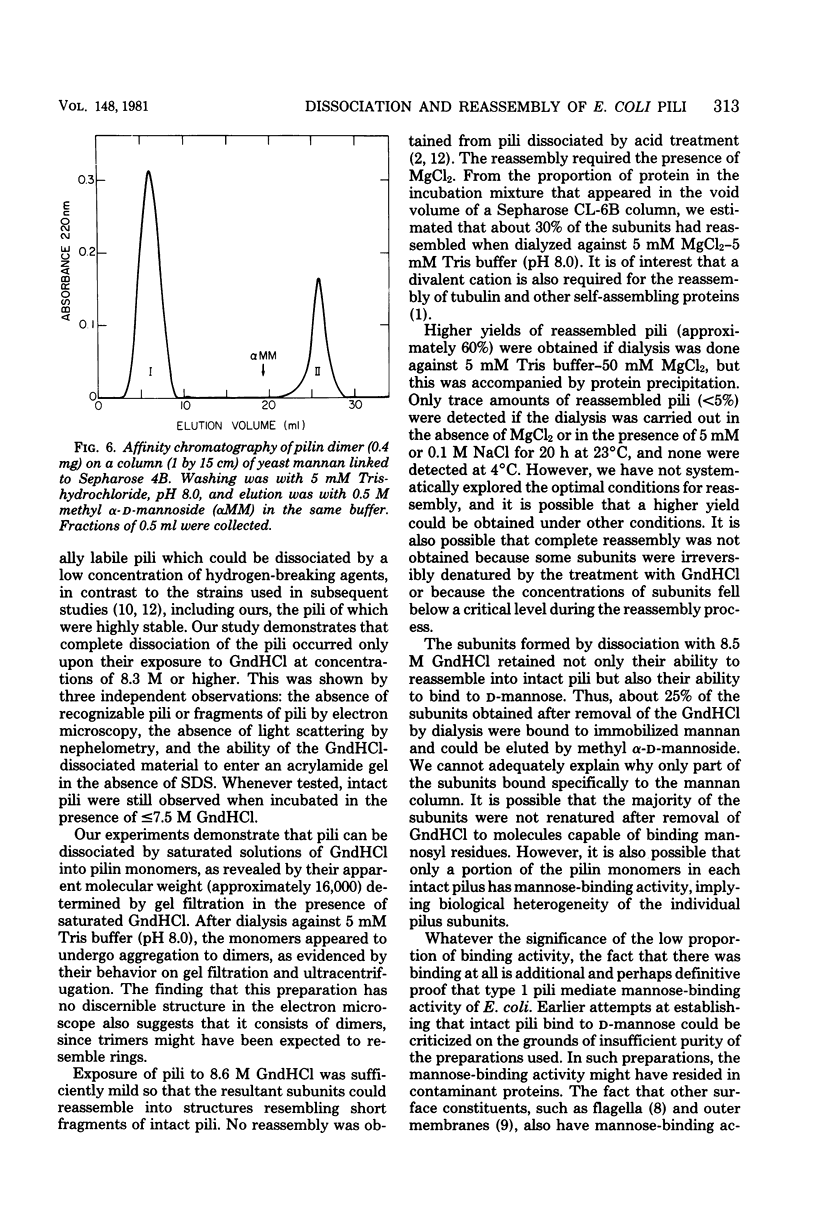
Abstract
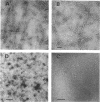
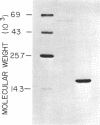
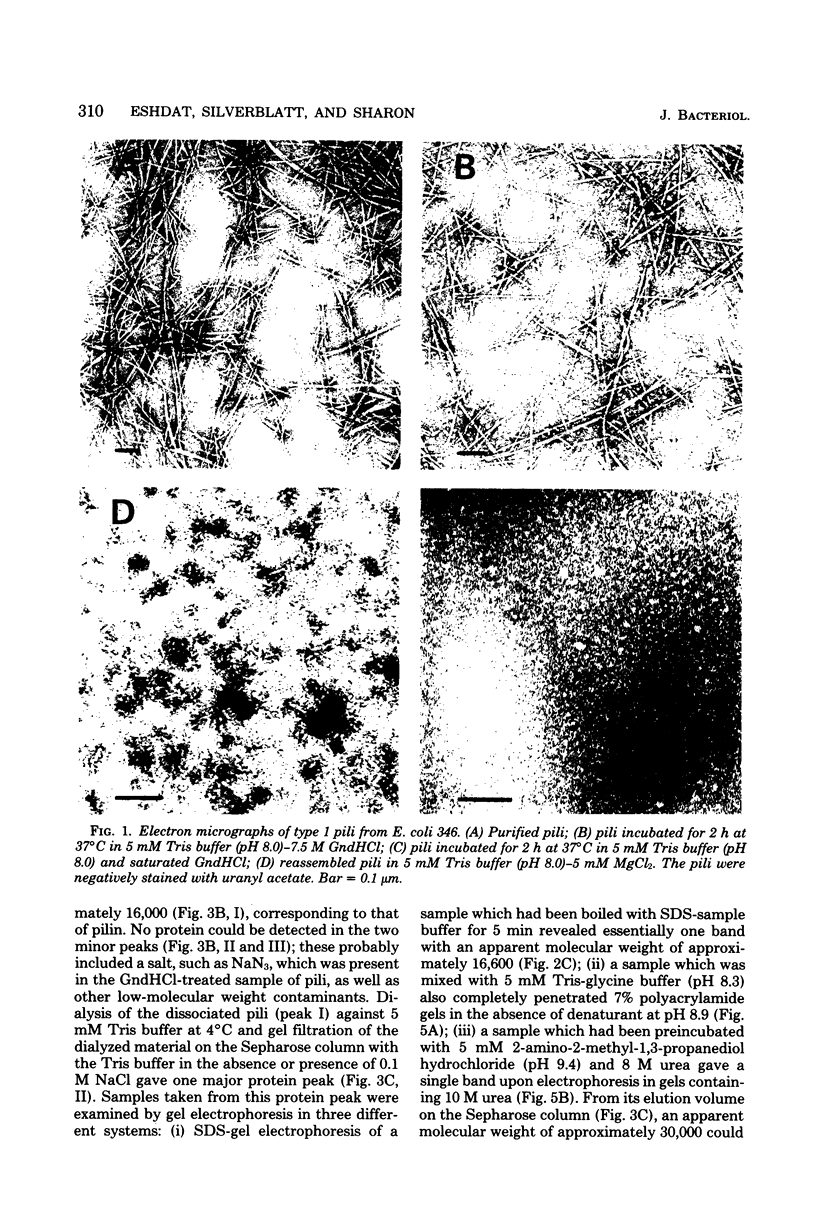
Escherichia coli type 1 pili, which mediate the mannose-sensitive adherence of the bacterium to eucaryotic cells, are comprised of very stable arrays of pilin protein subunits (molecular weight, approximately 17,000). Previous methods for the dissociation of pili caused their irreversible denaturation. We have found that incubation of pili in saturated guanidine hydrochloride at 37 degrees C led to their complete dissociation, as evidenced by nephelometry and electron microscopy. Gel chromatography of the dissociated pili on a Sepharose CL-6B column in the presence of saturated guanidine hydrochloride yielded a single protein peak with a molecular weight corresponding to that of pilin. Dialysis of this peak against 5 mM tris(hydroxymethyl)aminomethane hydrochloride (pH 8.0) and rechromatography in the same buffer afforded a major protein peak, probably consisting of pilin dimers. About 25% of the protein in this peak bound to a mannan-sepharose column and could be eluted with methyl alpha-D-mannoside. The pilin dimer gave a single protein band upon polyacrylamide gel electrophoresis in the presence of 0.1% sodium dodecyl sulfate (molecular weight, 16,600) or 10 M urea and penetrated completely into 7% gels in the absence of denaturants. Reassembly of the pilin dimers into pili was achieved upon dialysis against the tris(hydroxymethyl)aminomethane buffer containing 5 mM MgCl2, as observed by electron microscopy. Thus, the conditions used allow renaturation of the dissociated subunits and may aid in further studies of the structure-function relationship of pili.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINTON C. C., Jr, BUZZELL A., LAUFFER M. A. Electrophoresis and phage susceptibility studies on a filament-producing variant of the E. coli B bacterium. Biochim Biophys Acta. 1954 Dec;15(4):533–542. doi: 10.1016/0006-3002(54)90011-6. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Marcum J. M., Olmsted J. B., Murphy D. B., Johnson K. A. Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann N Y Acad Sci. 1975 Jun 30;253:107–132. doi: 10.1111/j.1749-6632.1975.tb19196.x. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Ofek I., Yashouv-Gan Y., Sharon N., Mirelman D. Isolation of a mannose-specific lectin from Escherichia coli and its role in the adherence of the bacteria to epithelial cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1551–1559. doi: 10.1016/0006-291x(78)91179-8. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Structure of common pili from Escherichia coli. J Bacteriol. 1979 Jun;138(3):969–975. doi: 10.1128/jb.138.3.969-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Altmann G., Eshdat Y. Screening of bacterial isolates for mannose-specific lectin activity by agglutination of yeasts. J Clin Microbiol. 1980 Apr;11(4):328–331. doi: 10.1128/jcm.11.4.328-331.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Cohen L. S. Antipili antibody affords protection against experimental ascending pyelonephritis. J Clin Invest. 1979 Jul;64(1):333–336. doi: 10.1172/JCI109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J. Ultraviolet irradiation disrupts somatic pili structure and function. Infect Immun. 1979 Sep;25(3):1060–1065. doi: 10.1128/iai.25.3.1060-1065.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]