Abstract
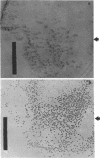
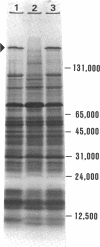
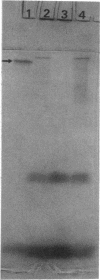
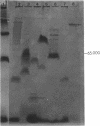
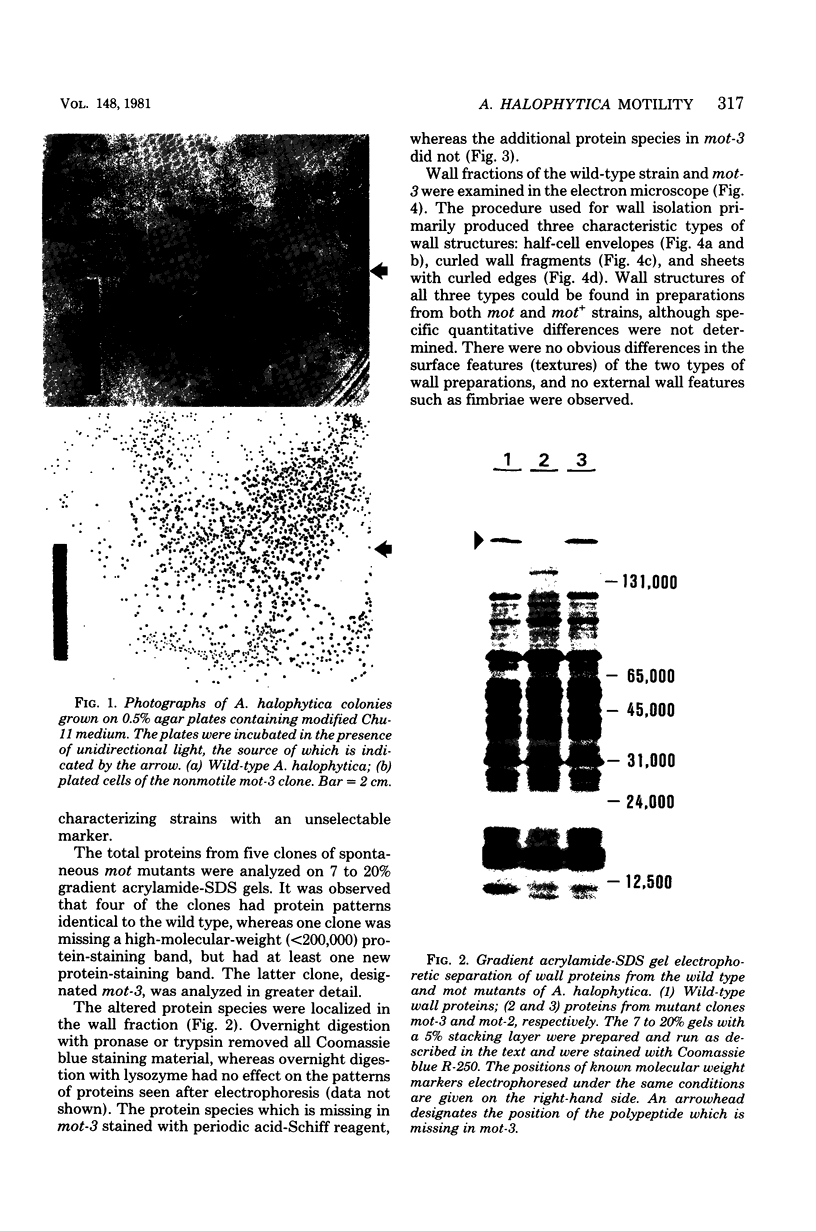
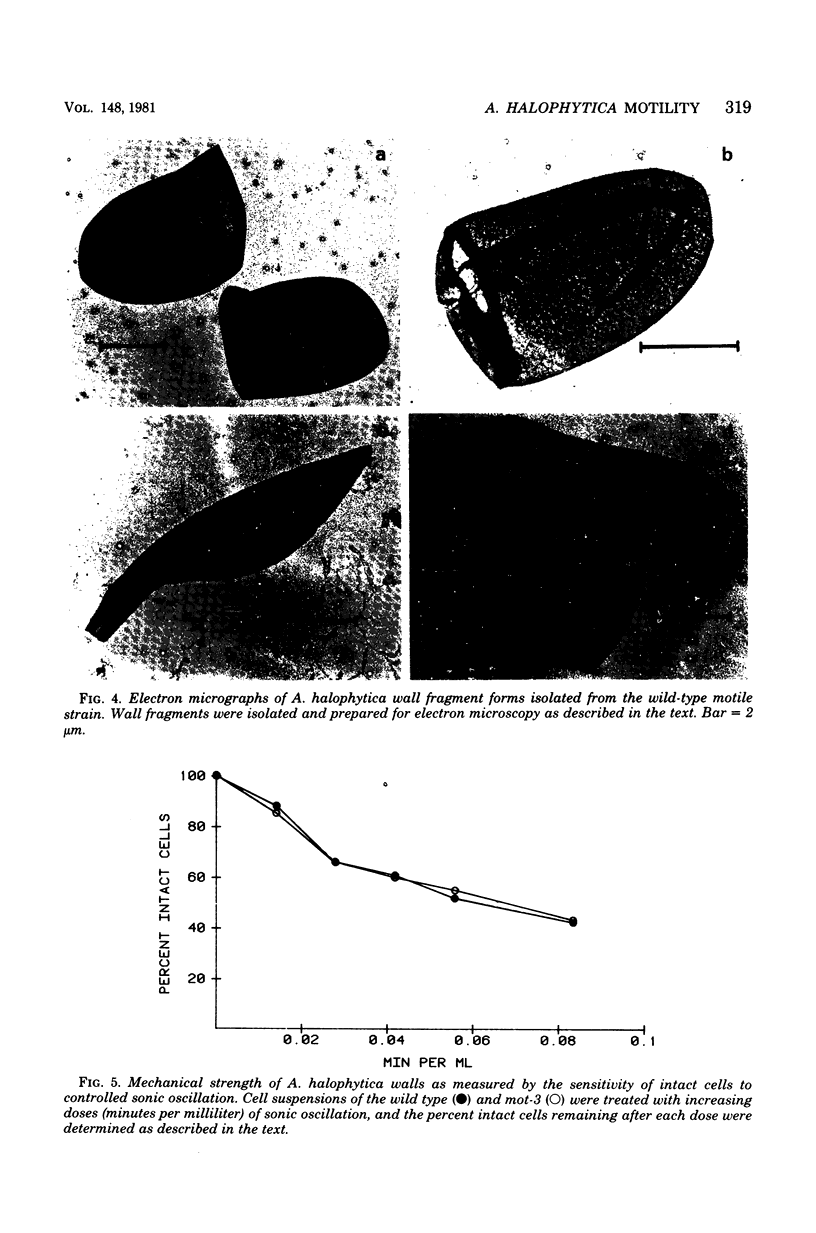
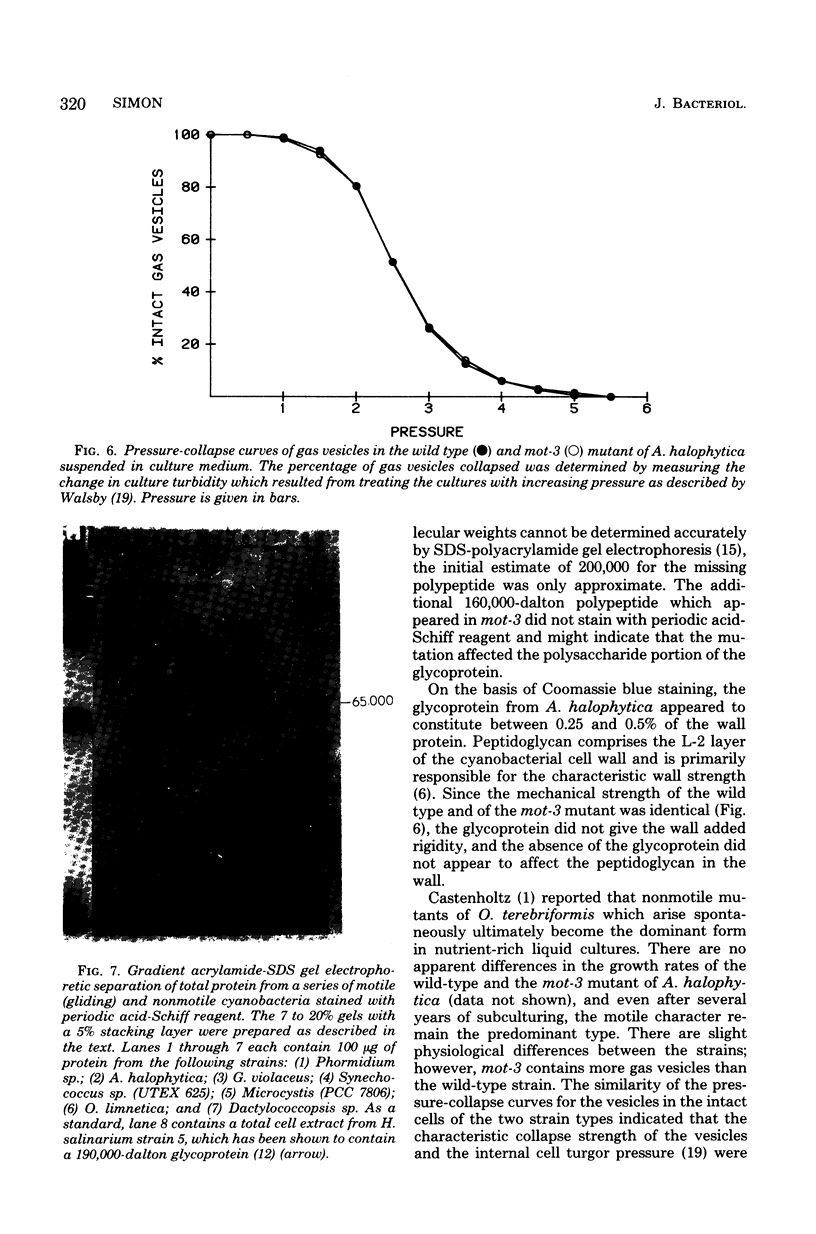
The unicellular cyanobacterium Aphanothece halophytica (PCC 7418) is motile, and spontaneous nonmotile (mot) mutants accumulate when the organism is subcultured. Analysis of mot mutants suggests that a glycoprotein in the cell wall is involved in the motility mechanism. Proteins from the wall fraction of the wild type and five mot clones were analyzed by gradient sodium dodecyl sulfate-acrylamide gel electrophoresis. Four clones were similar to the wild type, and one clone, mot-3, was missing a high-molecular-weight protein (approximately 200,000) and had at least one new polypeptide (160,000). The high-molecular-weight protein stained with periodic acid-Schiff reagent, suggesting that it was a glycoprotein. The absence of the protein in mot-3 did not affect the mechanical strength of the wall, since both mot-3 and wild-type cells were broken at the same rate by controlled cavitation. Several other cyanobacteria were also screened for the presence of glycoproteins. All motile strains have such proteins, although none had an apparent molecular weight as high as that in Aphanothece sp. Some motile strains, such as Oscillatoria limnetica and Phormidium sp., showed very large amounts of glycoproteins; whereas some nonmotile strains, such as Synechococcus sp. (UTEX 625) and Microcystis sp. (PCC 7820), showed no high-molecular-weight glycoproteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen Y., Padan E., Shilo M. Facultative anoxygenic photosynthesis in the cyanobacterium Oscillatoria limnetica. J Bacteriol. 1975 Sep;123(3):855–861. doi: 10.1128/jb.123.3.855-861.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch R. N., Sjoblad R. D. Flagellar structure and function in eubacteria. Annu Rev Microbiol. 1980;34:69–108. doi: 10.1146/annurev.mi.34.100180.000441. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacRae T. H., Dobson W. J., McCurdy H. D. Fimbriation in gliding bacteria. Can J Microbiol. 1977 Aug;23(8):1096–1108. doi: 10.1139/m77-165. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Purification and characterization of a prokaryotic glucoprotein from the cell envelope of Halobacterium salinarium. J Biol Chem. 1976 Apr 10;251(7):2005–2014. [PubMed] [Google Scholar]
- Slater G. G. Stable pattern formation and determination of molecular size by pore-limit electrophoresis. Anal Chem. 1969 Jul;41(8):1039–1041. doi: 10.1021/ac60277a003. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]