Abstract
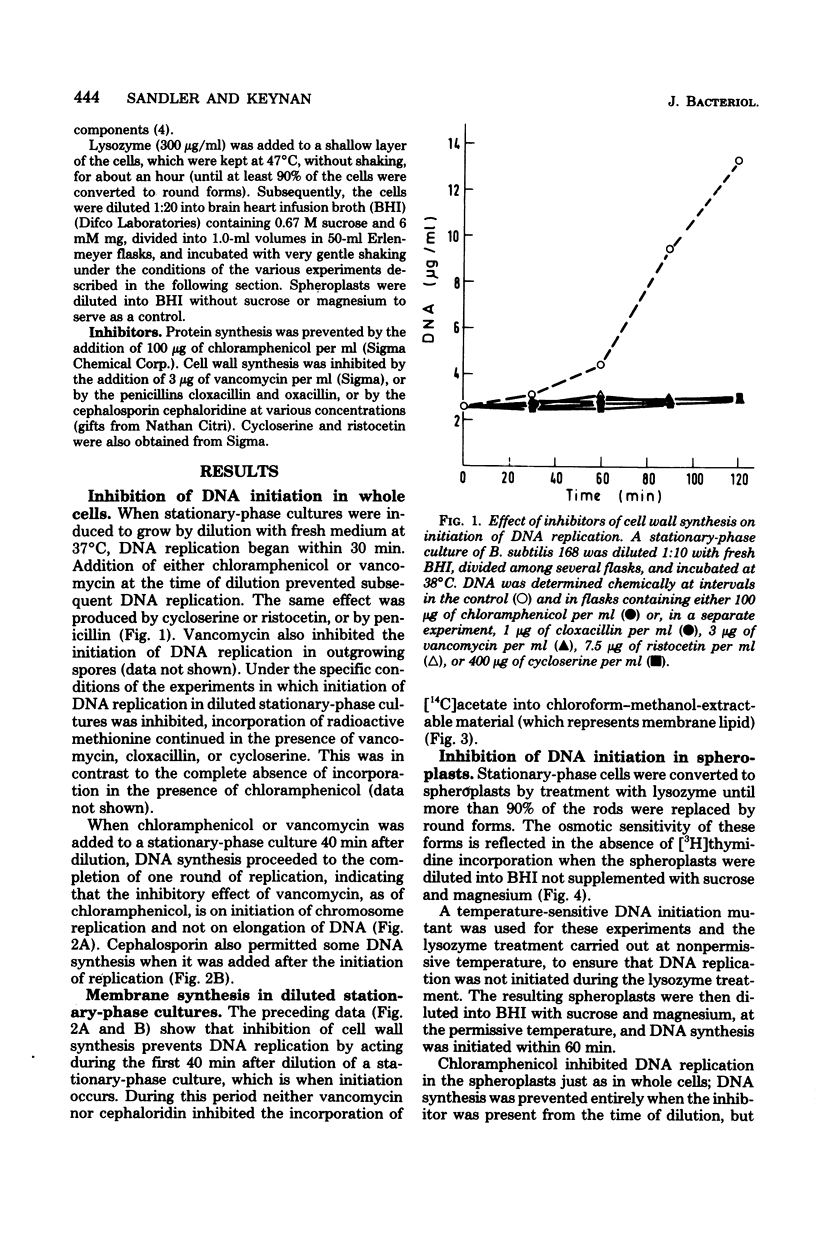
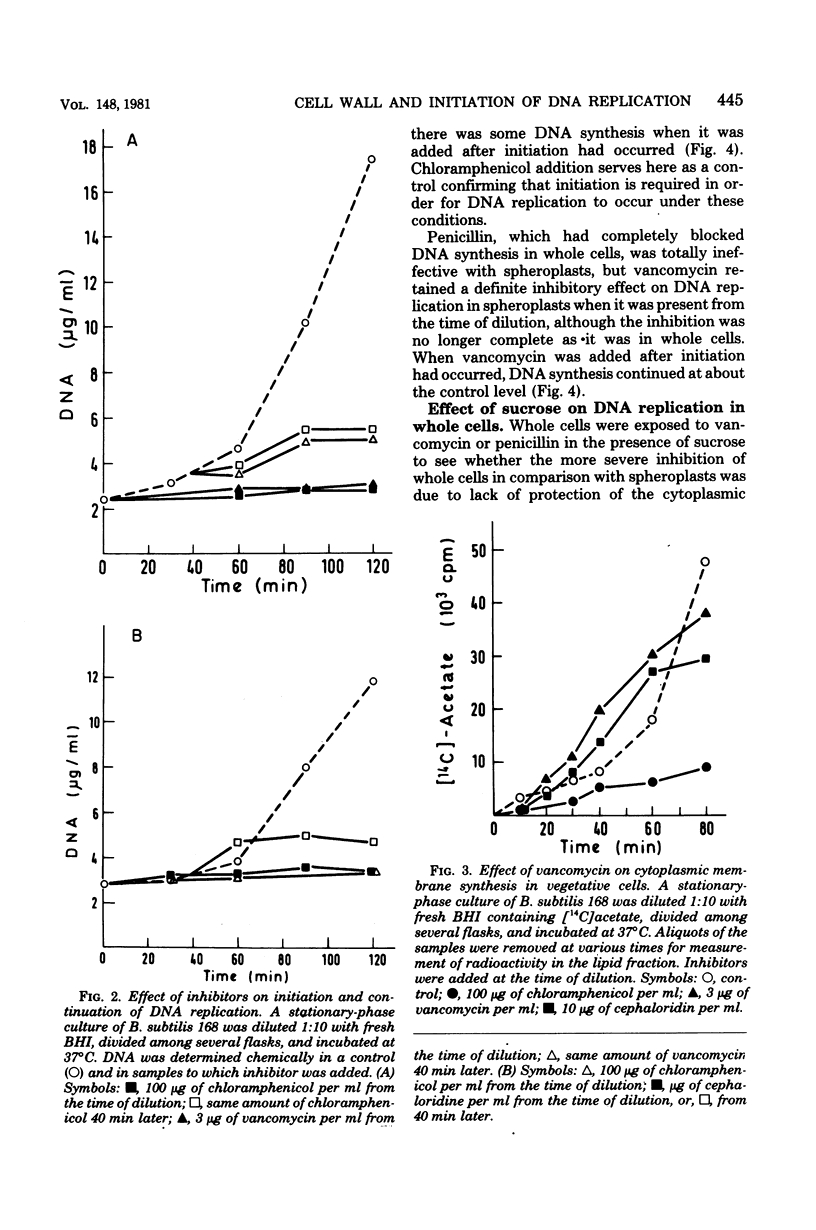
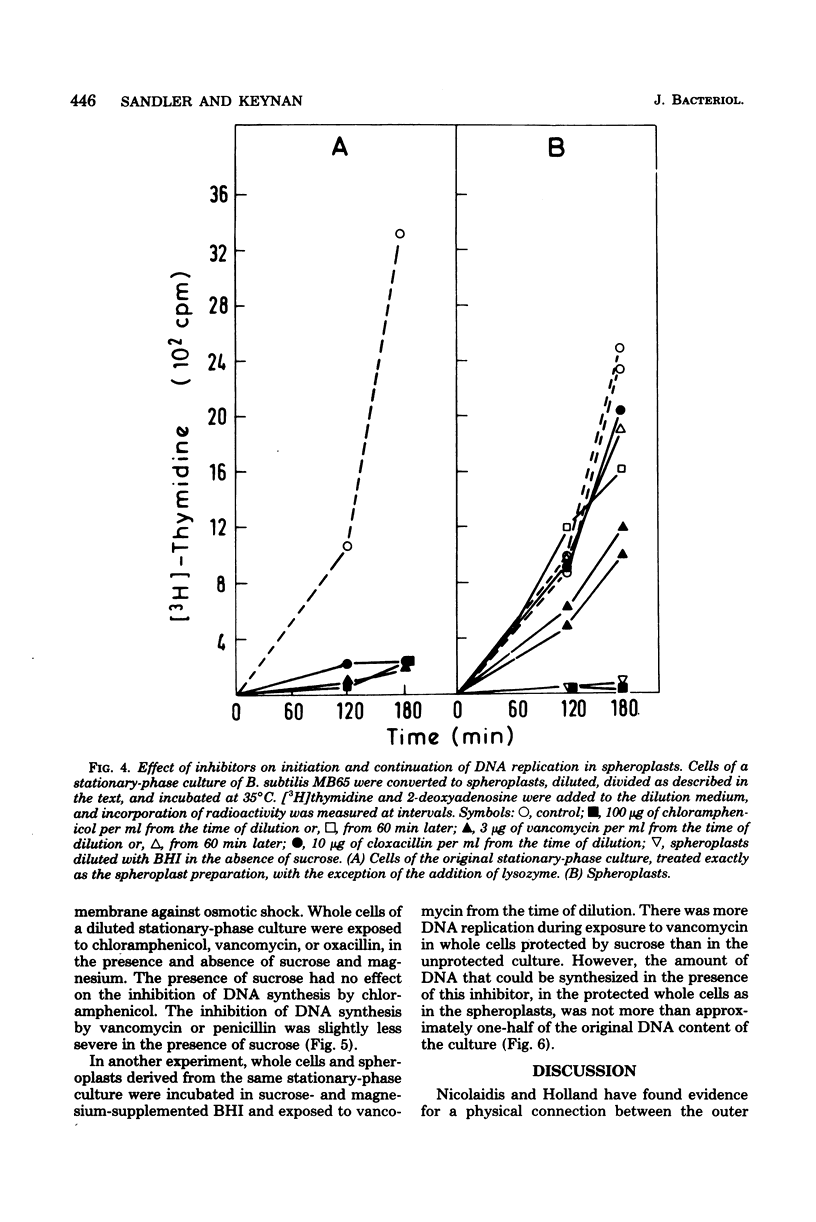
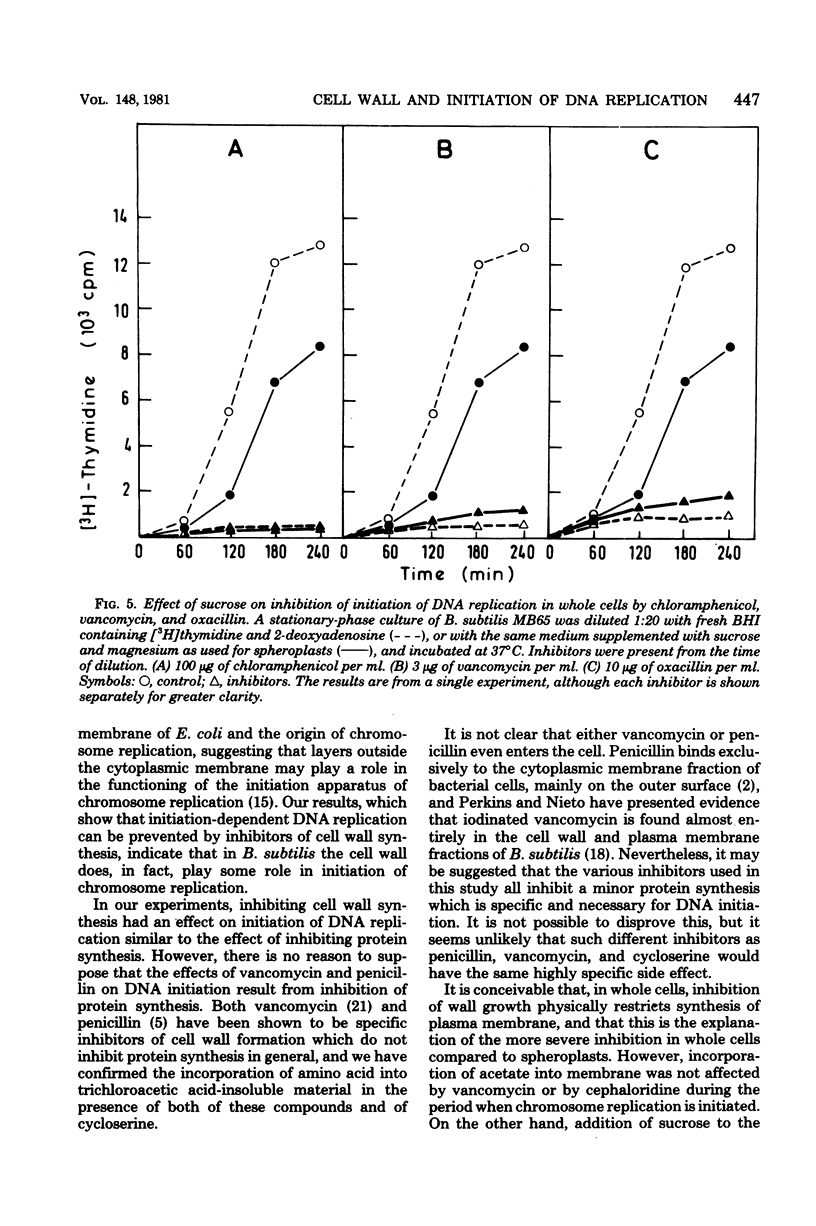
We have observed a connection between cell wall synthesis and the initiation of chromosome replication in Bacillus subtilis. Initiation of chromosome replication was prevented in synchronous cultures in the presence of the cell wall synthesis inhibitor vancomycin. When vancomycin was added to the cultures after initiation of chromosome replication, one round of replication was completed but no reinitiation occurred. Similar results were obtained when cell wall synthesis was inhibited by ristocetin, cycloserine, cloxacillin, or cephaloridine. When sucrose was added to the medium, initiation of deoxyribonucleic acid replication occurred in the presence of vancomycin, to an extent which allowed replication of no more than approximately one-half of the deoxyribonucleic acid of the culture. The same was found in cultures of spheroplasts of B. subtilis. However, initiation of chromosome replication in spheroplasts was completely insensitive to cloxacillin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. G., Rutberg L., Samuelsson B. The chemical composition of the cytoplasmic membrane of Bacillus subtilis. Eur J Biochem. 1967 Nov;2(4):448–453. doi: 10.1111/j.1432-1033.1967.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER P. D. The site of action of penicillin: some changes in Staphylococcus aureus during the first two hours growth in penicillin media. J Gen Microbiol. 1955 Aug;13(1):22–38. doi: 10.1099/00221287-13-1-22. [DOI] [PubMed] [Google Scholar]
- Clive D., Landman O. E. Reversion of Bacillus subtilis protoplasts to the bacillary form induced by exogenous cell wall, bacteria and by growth in membrane filters. J Gen Microbiol. 1970 May;61(2):233–243. doi: 10.1099/00221287-61-2-233. [DOI] [PubMed] [Google Scholar]
- Fielding P., Fox C. F. Evidence for stable attachment of DNA to membrane at the replication origin of Escherichia coli. Biochem Biophys Res Commun. 1970 Oct 9;41(1):157–162. doi: 10.1016/0006-291x(70)90482-1. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. Initiation of chromosome replication in Escherichia coli. I. Requirements for RNA and protein synthesis at different growth rates. J Mol Biol. 1974 Mar 25;84(1):1–19. doi: 10.1016/0022-2836(74)90209-5. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. Initiation of chromosome replication in Escherichia coli. II. Analysis of the control mechanism. J Mol Biol. 1974 Mar 25;84(1):21–36. doi: 10.1016/0022-2836(74)90210-1. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Karamata D., Gross J. D. Isolation and genetic analysis of temperature-sensitive mutants of B. subtilis defective in DNA synthesis. Mol Gen Genet. 1970;108(3):277–287. doi: 10.1007/BF00283358. [DOI] [PubMed] [Google Scholar]
- Nickerson K. W., De Pinto J., Bulla L. A., Jr Lipid metabolism during bacterial growth, sporulation, and germination: kinetics of fatty acid and macromolecular synthesis during spore germination and outgrowth of Bacillus thuringiensis. J Bacteriol. 1975 Jan;121(1):227–233. doi: 10.1128/jb.121.1.227-233.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaidis A. A., Holland I. B. Evidence for the specific association of the chromosomal origin with outer membrane fractions isolated from Escherichia coli. J Bacteriol. 1978 Jul;135(1):178–189. doi: 10.1128/jb.135.1.178-189.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M. A., Sueoka N. Membrane attachment of the replication origins of a multifork (dichotomous) chromosome in Bacillus subtilis. J Mol Biol. 1972 Aug 21;69(2):237–248. doi: 10.1016/0022-2836(72)90228-8. [DOI] [PubMed] [Google Scholar]
- Parker D. L., Glaser D. A. Chromosomal sites of DNA-membrane attachment in Escherichia coli. J Mol Biol. 1974 Aug 5;87(2):153–168. doi: 10.1016/0022-2836(74)90140-5. [DOI] [PubMed] [Google Scholar]
- Perkins H. R., Nieto M. The preparation of iodinated vancomycin and its distribution in bacteria treated with the antibiotic. Biochem J. 1970 Jan;116(1):83–92. doi: 10.1042/bj1160083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci O., Zuchowski C. Non-random segregation of DNA strands in Escherichia coli B-r. J Mol Biol. 1973 Nov 5;80(3):477–503. doi: 10.1016/0022-2836(73)90417-8. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Halvorson H. O. Method for restricting incorporation of radioactivity from 3 H-thymidine into deoxyribonucleic acid only during outgrowth of spores of Bacillus cereus T. J Bacteriol. 1972 Feb;109(2):599–605. doi: 10.1128/jb.109.2.599-605.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E. Peptidoglycan synthesis in bacilli. II. Characteristics of protoplast membrane preparations. Biochim Biophys Acta. 1971 May 18;237(2):255–272. doi: 10.1016/0304-4165(71)90316-3. [DOI] [PubMed] [Google Scholar]
- Sandler N., Keynan A. Changes in sporulation potential during the growth cycle of Bacillus subtilis. Arch Microbiol. 1979 Oct;123(1):9–14. doi: 10.1007/BF00403497. [DOI] [PubMed] [Google Scholar]
- Snyder R. W., Young F. E. Association between the chromosome and the cytoplasmic membrane in Bacillus subtilis. Biochem Biophys Res Commun. 1969 May 8;35(3):354–362. doi: 10.1016/0006-291x(69)90506-3. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Hammers J. M. Isolation of DNA-membrane complex in Bacillus subtilis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4787–4791. doi: 10.1073/pnas.71.12.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Quinn W. G. Membrane attachment of the chromosome replication origin in Bacillus subtilis. Cold Spring Harb Symp Quant Biol. 1968;33:695–705. doi: 10.1101/sqb.1968.033.01.078. [DOI] [PubMed] [Google Scholar]
- Wada C., Yura T. Phenethyl alcohol resistance in Escherichia coli. 3. A temperature-sensitive mutation(dnaP) affecting DNA replication. Genetics. 1974 Jun;77(2):199–220. doi: 10.1093/genetics/77.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston S., Sueoka N. DNA-membrane association is necessary for initiation of chromosomal and plasmid replication in Bacillus subtilis. Proc Natl Acad Sci U S A. 1980 May;77(5):2834–2838. doi: 10.1073/pnas.77.5.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Murakami S., Yoshikawa H. Chromosome-membrane association in Bacillus subtilis. I. DNA release from membrane fraction. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1559–1565. doi: 10.1016/s0006-291x(71)80264-4. [DOI] [PubMed] [Google Scholar]


