Abstract
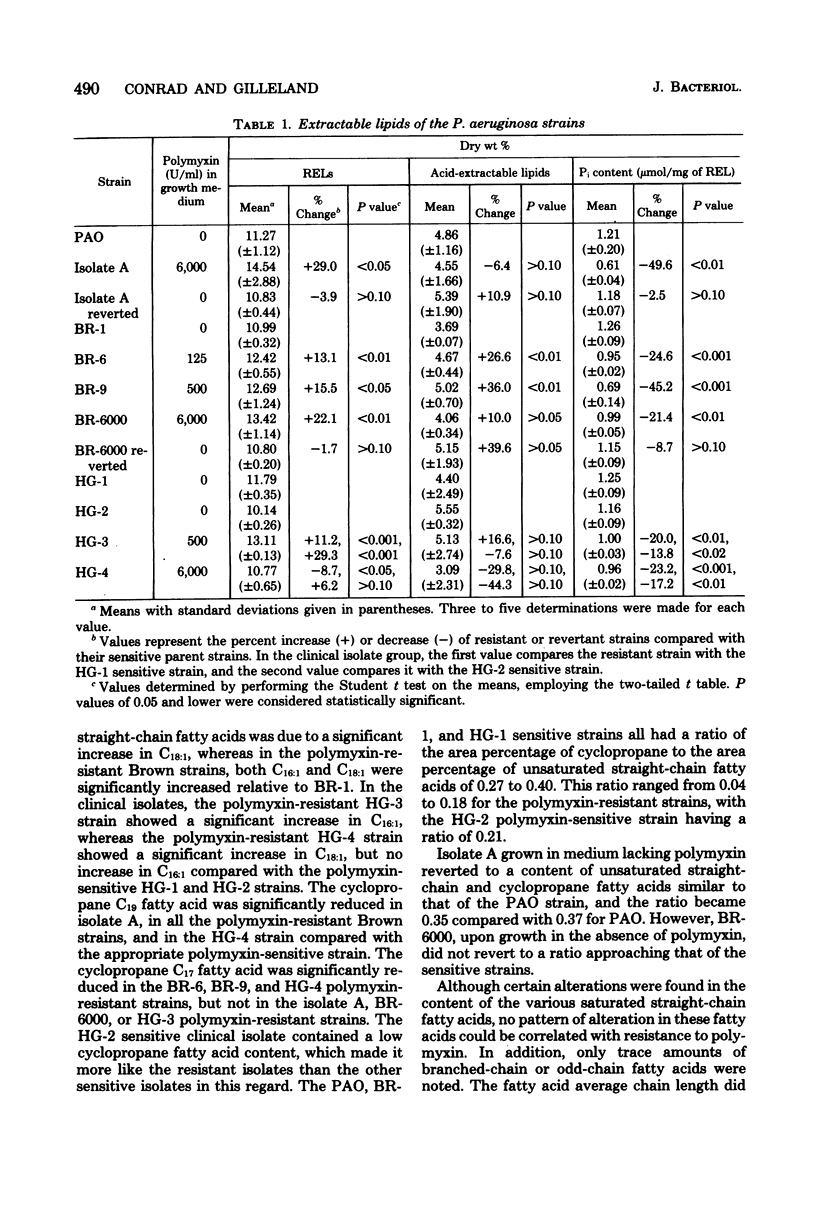
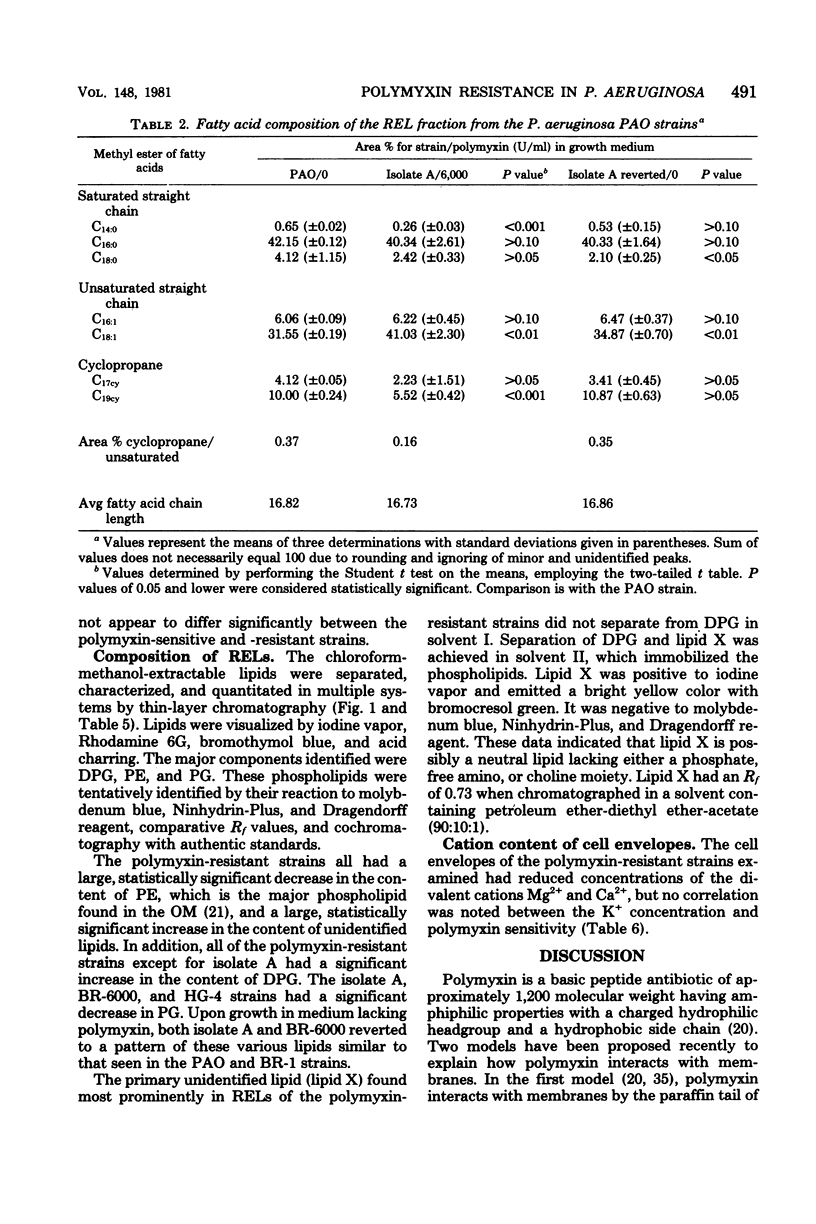
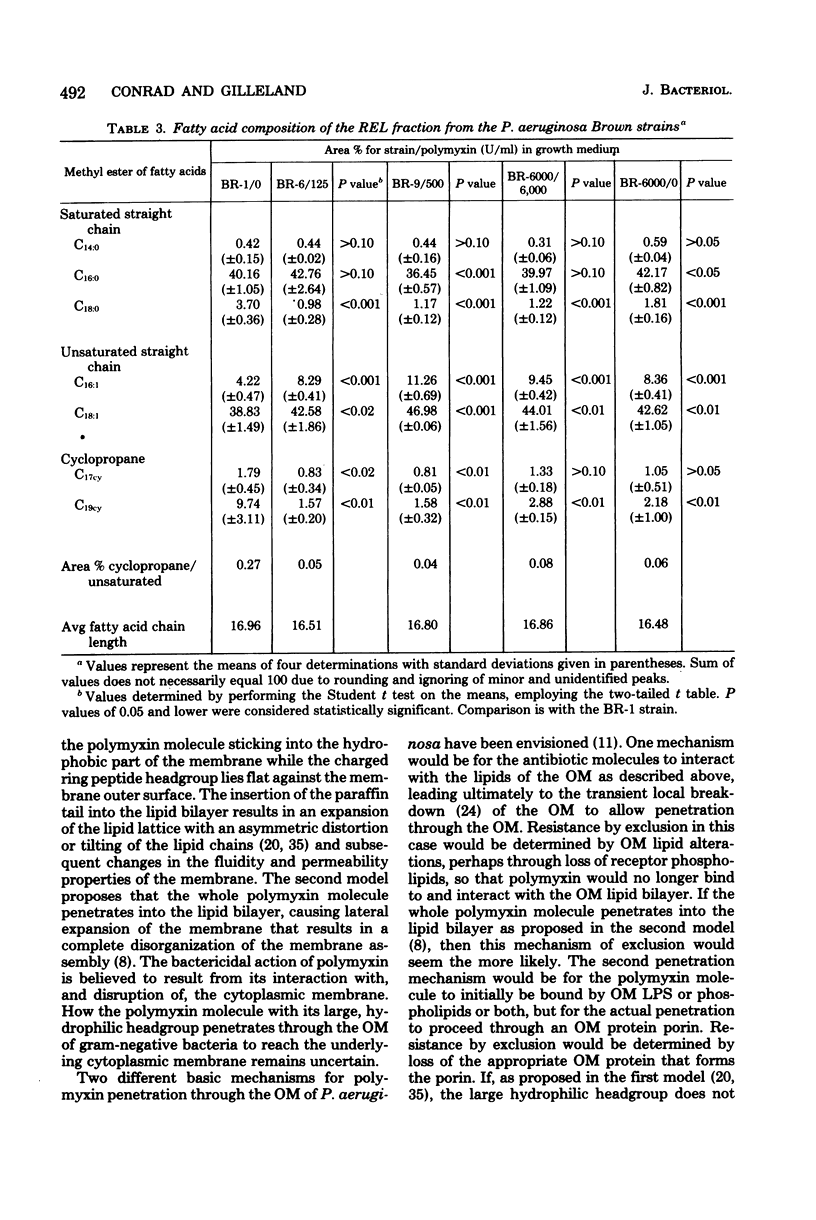
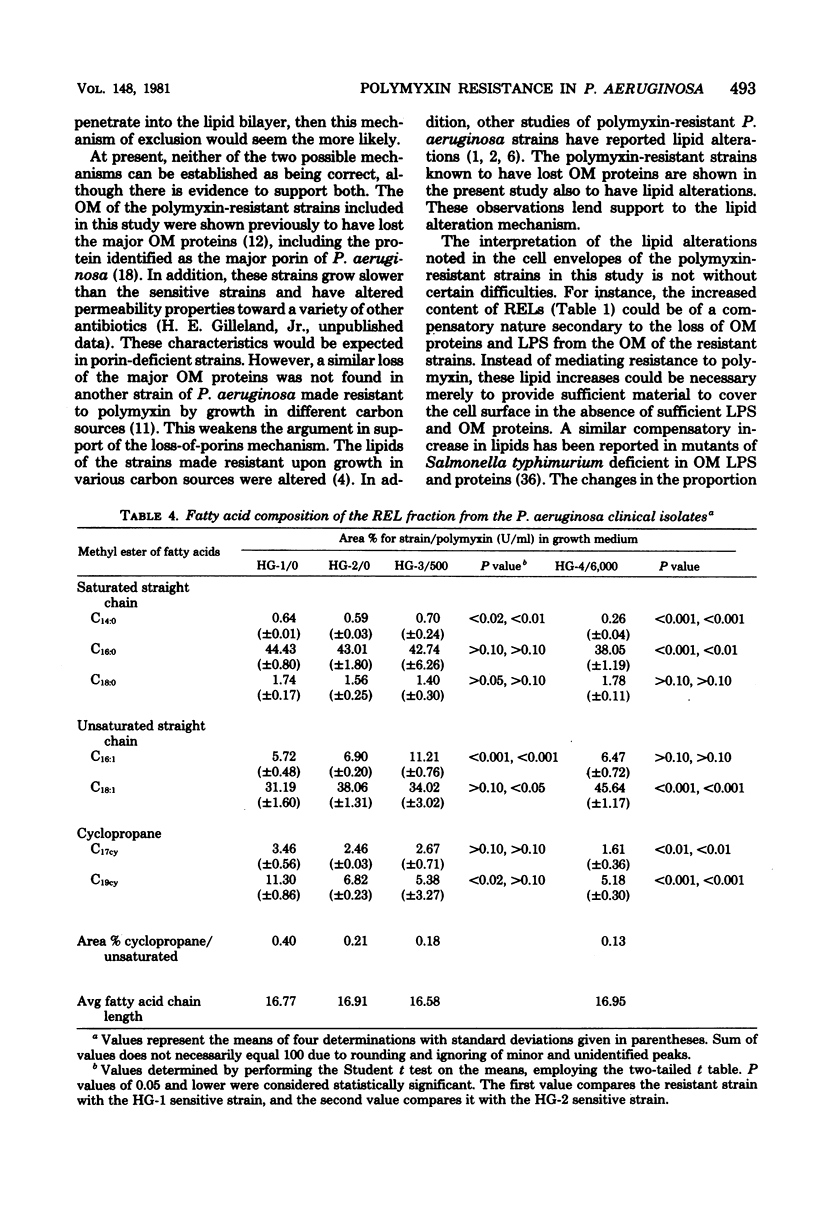
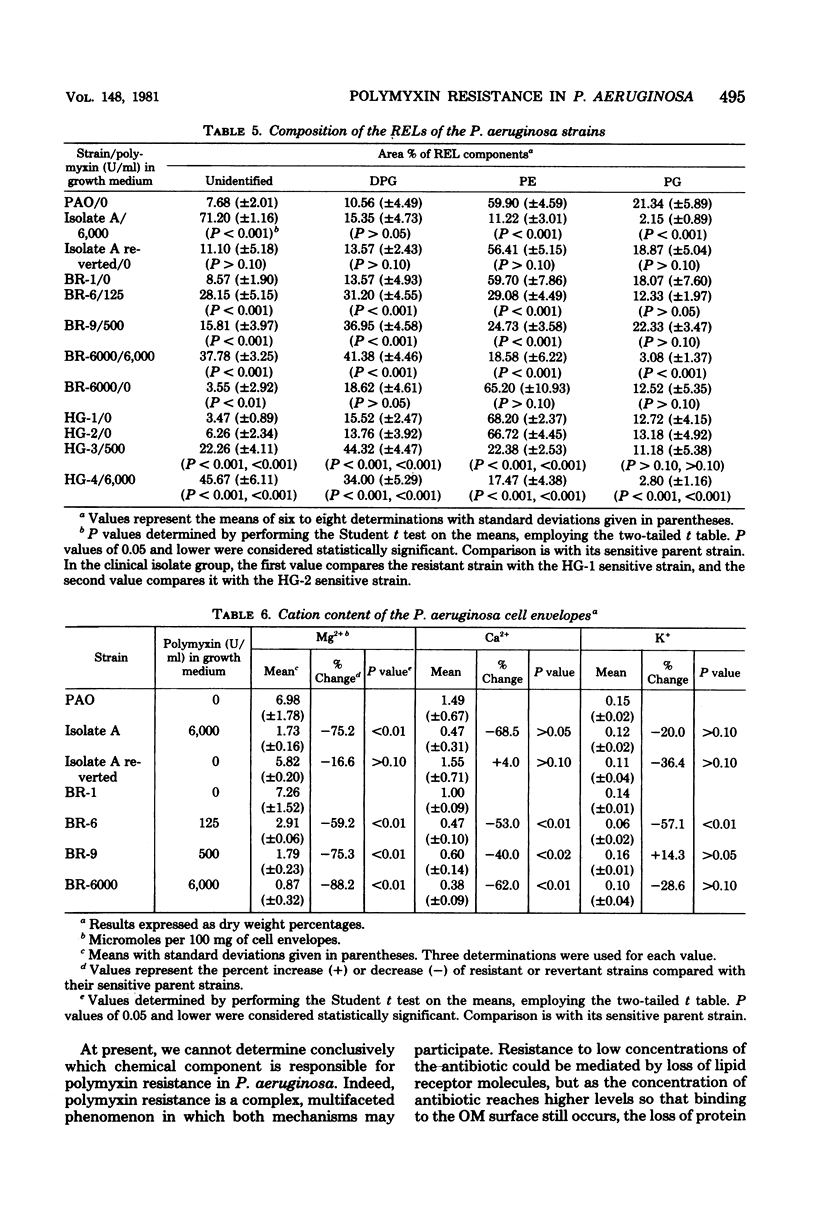
The lipid composition of cells of Pseudomonas aeruginosa strains resistant to polymyxin was compared with the lipid composition of cells of polymyxin-sensitive strains as to their content of readily extractable lipids (RELs), acid-extractable lipids, the fatty acid composition of RELs, and the contents of various phospholipids in the REL fraction. The polymyxin-resistant strains had an increased content of RELs, but a decreased phospholipid content. The REL fraction from the polymyxin-resistant strains had an increased content of unsaturated fatty acids accompanied by a decreased content of cyclopropane fatty acids as compared with the fatty acid composition of RELs from polymyxin-sensitive strains. The phosphatidylethanolamine content was greatly reduced in the polymyxin-resistant strains, whereas the content of an unidentified lipid, thought to be a neutral lipid lacking either a phosphate, free amino, or choline moiety, was greatly increased. Cell envelopes of the polymyxin-resistant strains contained reduced concentrations of Mg2+ and Ca2+ as compared with the cell envelopes of polymyxin-sensitive strains. It appears that polymyxin resistance in these strains is associated with a significant alteration in the lipid composition and divalent cation content of the cell envelope.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. R., Watkins W. M. Low magnesium and phospholipid content of cell wals of Pseudomonas aeruginosa resistant to polymyxin. Nature. 1970 Sep 26;227(5265):1360–1361. doi: 10.1038/2271360a0. [DOI] [PubMed] [Google Scholar]
- Conrad R. S., Wulf R. G., Clay D. L. Effects of carbon sources on antibiotic resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1979 Jan;15(1):59–66. doi: 10.1128/aac.15.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame J. B., Shapiro B. M. Lipid and lipopolysaccharide composition of Escherichia coli surface-altered mutants selected for resistance to levallorphan, tetracaine, and polymyxin. J Bacteriol. 1979 Feb;137(2):1043–1047. doi: 10.1128/jb.137.2.1043-1047.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrer E., Teuber M. Induction of polymyxin resistance in Pseudomonas fluorescens by phosphate limitation. Arch Microbiol. 1977 Jul 26;114(1):87–89. doi: 10.1007/BF00429636. [DOI] [PubMed] [Google Scholar]
- Dunnick J. K., O'Leary W. M. Correlation of bacteria lipid composition with antibiotic resistance. J Bacteriol. 1970 Mar;101(3):892–900. doi: 10.1128/jb.101.3.892-900.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mashak E. M., Tocanne J. F. Polymyxin B-phosphatidylglycerol interactions. A monolayer (pi, delta V) study. Biochim Biophys Acta. 1980 Feb 28;596(2):165–179. doi: 10.1016/0005-2736(80)90351-x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Conrad R. S. Effects of carbon sources on chemical composition of cell envelopes of Pseudomonas aeruginosa in association with polymyxin resistance. Antimicrob Agents Chemother. 1980 Apr;17(4):623–628. doi: 10.1128/aac.17.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Lyle R. D. Chemical alterations in cell envelopes of polymyxin-resistant Pseudomonas aeruginosa isolates. J Bacteriol. 1979 Jun;138(3):839–845. doi: 10.1128/jb.138.3.839-845.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Murray R. G. Demonstration of cell division by septation in a variety of gram-negative rods. J Bacteriol. 1975 Feb;121(2):721–725. doi: 10.1128/jb.121.2.721-725.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Murray R. G. Ultrastructural study of polymyxin-resistant isolates of Pseudomonas aeruginosa. J Bacteriol. 1976 Jan;125(1):267–281. doi: 10.1128/jb.125.1.267-281.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Eagon R. G. Ultrastructural and chemical alteration of the cell envelope of Pseudomonas aeruginosa, associated with resistance to ethylenediaminetetraacetate resulting from growth in a Mg2+-deficient medium. J Bacteriol. 1974 Jan;117(1):302–311. doi: 10.1128/jb.117.1.302-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Roth I. L., Eagon R. G. Freeze-etch study of Pseudomonas aeruginosa: localization within the cell wall of an ethylenediaminetetraacetate-extractable. J Bacteriol. 1973 Jan;113(1):417–432. doi: 10.1128/jb.113.1.417-432.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D. The activity of polymyxins against dense populations of Escherichia coli. J Gen Microbiol. 1975 Nov;91(1):110–118. doi: 10.1099/00221287-91-1-110. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Decad G. M., Nikaido H. Identification of the protein producing transmembrane diffusion pores in the outer membrane of Pseudomonas aeruginosa PA01. Biochim Biophys Acta. 1979 Jul 5;554(2):323–331. doi: 10.1016/0005-2736(79)90373-0. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Irvin R. T., Costerton J. W., Carey A. M. Pseudomonas aeruginosa outer membrane: peptidoglycan-associated proteins. J Bacteriol. 1981 Jan;145(1):628–631. doi: 10.1128/jb.145.1.628-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann W., Galla H. J., Sackmann E. Polymyxin binding to charged lipid membranes. An example of cooperative lipid-protein interaction. Biochim Biophys Acta. 1978 Jun 16;510(1):124–139. doi: 10.1016/0005-2736(78)90135-9. [DOI] [PubMed] [Google Scholar]
- Koike M., Iida K., Matsuo T. Electron microscopic studies on mode of action of polymyxin. J Bacteriol. 1969 Jan;97(1):448–452. doi: 10.1128/jb.97.1.448-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry R. R., Tinsley I. J. A simple, sensitive method for lipid phosphorus. Lipids. 1974 Jul;9(7):491–492. doi: 10.1007/BF02534277. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Miller I. R., Bach D., Teuber M. Effect of polymyxin B on the structure and the stability of lipid layers. J Membr Biol. 1978 Feb 6;39(1):49–56. doi: 10.1007/BF01872754. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980 Aug;143(2):872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Rogers S. W., Gilleland H. E., Jr, Eagon R. G. Characterization of a protein-lipopolysaccharide complex released from cell walls of Pseudomonas aeruginosa by ethylenediaminetetraacetic acid. Can J Microbiol. 1969 Jul;15(7):743–748. doi: 10.1139/m69-130. [DOI] [PubMed] [Google Scholar]
- Schindler P. R., Teuber M. Action of polymyxin B on bacterial membranes: morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli B. Antimicrob Agents Chemother. 1975 Jul;8(1):95–104. doi: 10.1128/aac.8.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Iyobe S., Mitsuhashi S. Inducible high resistance to colistin in Proteus strains. Antimicrob Agents Chemother. 1977 Jul;12(1):1–3. doi: 10.1128/aac.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixl F., Galla H. J. Cooperative lipid-protein interaction. Effect of pH and ionic strength on polymyxin binding to phosphatidic acid membranes. Biochim Biophys Acta. 1979 Nov 2;557(2):320–330. doi: 10.1016/0005-2736(79)90330-4. [DOI] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnett J. D., Gilleland H. E., Jr, Eagon R. G. Proteins released from cell envelopes of Pseudomonas aeruginosa on exposure to ethylenediaminetetraacetate: comparison with dimethylformamide-extractable proteins. J Bacteriol. 1973 Apr;114(1):399–407. doi: 10.1128/jb.114.1.399-407.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Mechanism of polymyxin B resistance in Proteus mirabilis. J Bacteriol. 1970 Oct;104(1):289–294. doi: 10.1128/jb.104.1.289-294.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber M., Bader J. Action of polymyxin B on bacterial membranes. Binding capacities for polymyxin B of inner and outer membranes isolated from Salmonella typhimurium G30. Arch Microbiol. 1976 Aug;109(1-2):51–58. doi: 10.1007/BF00425112. [DOI] [PubMed] [Google Scholar]
- Tsang J. C., Weber D. A., Brown D. A. Evidences for complex formation between polymyxin B and lipopolysaccharides from Serratia marcescens. J Antibiot (Tokyo) 1976 Jul;29(7):735–742. doi: 10.7164/antibiotics.29.735. [DOI] [PubMed] [Google Scholar]
- Verkleij A., van Alphen L., Bijvelt J., Lugtenberg B. Architecture of the outer membrane of Escherichia coli K12. II. Freeze fracture morphology of wild type and mutant strains. Biochim Biophys Acta. 1977 Apr 18;466(2):269–282. doi: 10.1016/0005-2736(77)90224-3. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Verkleij A., Leunissen-Bijvelt J., Lugtenberg B. Architecture of the outer membrane of Escherichia coli. III. Protein-lipopolysaccharide complexes in intramembraneous particles. J Bacteriol. 1978 Jun;134(3):1089–1098. doi: 10.1128/jb.134.3.1089-1098.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



