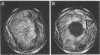
Abstract
A mutant, cyaR1, affecting regulation of adenylate cyclase expression or activity is described. It was obtained as a thermoresistant revertant of a strain harboring a thermosensitive transcription termination factor, rho (rho-15). This mutant failed to synthesize adenosine 3',5'-phosphate and exhibited a carbohydrate-negative phenotype. A secondary mutation at the crp locus (crpC) restored the ability of the mutant to synthesize adenosine 3',5'-phosphate, enabled the expression of catabolite-sensitive operons, and conferred on the strain an extreme sensitivity to catabolite repression. In addition, we showed that the crpC mutation restored the pleiotropic carbohydrate-positive phenotype even in a delta cya background. We interpret this to mean that the adenosine 3',5'-phosphate receptor protein regulates negatively either the activity or synthesis of adenylate cyclase and that the cyaR1 mutation is either in a regulatory protein or a regulatory site of adenylate cyclase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. K. Suppression of defects in cyclic adenosine 3',5'-monophosphate metabolism in Escherichia coli. J Bacteriol. 1980 Oct;144(1):205–209. doi: 10.1128/jb.144.1.205-209.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L., Drexler M. The cyclic 3',5'-adenosine monophosphate receptor protein and regulation of cyclic 3',5'-adenosine monophosphate synthesis in Escherichia coli. Mol Gen Genet. 1978 Sep 20;165(1):47–56. doi: 10.1007/BF00270375. [DOI] [PubMed] [Google Scholar]
- Brickman E., Soll L., Beckwith J. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J Bacteriol. 1973 Nov;116(2):582–587. doi: 10.1128/jb.116.2.582-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin A., Dondon L., Joseph E., Ullmann A. Transcription-translation coupling and polarity: a possible role of cyclic AMP. Biochimie. 1981;63(5):419–424. doi: 10.1016/s0300-9084(81)80015-6. [DOI] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessein A., Schwartz M., Ullmann A. Catabolite repression in Escherichia coli mutants lacking cyclic AMP. Mol Gen Genet. 1978 Jun 1;162(1):83–87. doi: 10.1007/BF00333853. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Fraser A. D., Yamazaki H. Determination of the rates of synthesis and degradation of adenosine 3',5'-cyclic monophosphate in Escherichia coli CRP- and CRP+ strains. Can J Biochem. 1978 Sep;56(9):849–852. doi: 10.1139/o78-130. [DOI] [PubMed] [Google Scholar]
- Guidi-Rontani C., Danchin A., Ullmann A. Catabolite repression in Escherichia coli mutants lacking cyclic AMP receptor protein. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5799–5801. doi: 10.1073/pnas.77.10.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. P., Peterkofsky A. Glucose-sensitive adenylate cyclase in toluene-treated cells of Escherichia coli B. J Biol Chem. 1975 Jun 25;250(12):4656–4662. [PubMed] [Google Scholar]
- Inoko H., Shigesada K., Imai M. Isolation and characterization of conditional-lethal rho mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1162–1166. doi: 10.1073/pnas.74.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph E., Danchin A., Ullmann A. Regulation of galactose operon expression: glucose effects and role of cyclic adenosine 3',5'-monophosphate. J Bacteriol. 1981 Apr;146(1):149–154. doi: 10.1128/jb.146.1.149-154.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Glucose and the metabolism of adenosine 3':5'-cyclic monophosphate in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2794–2798. doi: 10.1073/pnas.68.11.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Interaction of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system with adenylate cyclase of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2920–2924. doi: 10.1073/pnas.72.8.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Effects of crp mutations on adenosine 3',5'-monophosphate metabolism in Salmonella typhimurium. J Bacteriol. 1976 Jul;127(1):120–127. doi: 10.1128/jb.127.1.120-127.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Danchin A. Restriction map of the cya region of the Escherichia coli K12 chromosome. Biochimie. 1981 Aug-Sep;63(8-9):719–722. doi: 10.1016/s0300-9084(81)80220-9. [DOI] [PubMed] [Google Scholar]
- Sabourin D., Beckwith J. Deletion of the Escherichia coli crp gene. J Bacteriol. 1975 Apr;122(1):338–340. doi: 10.1128/jb.122.1.338-340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Expression phénotypique et localisation génétique de mutations affectant le métabolisme du maltose chez Escherichia coli K 12. Ann Inst Pasteur (Paris) 1967 Jun;112(6):673–698. [PubMed] [Google Scholar]
- Suelter C. H., Wang J., Snell E. E. Direct spectrophotometric assay of tryptophanase. FEBS Lett. 1976 Jul 15;66(2):230–232. doi: 10.1016/0014-5793(76)80510-8. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Shibuya M., Kaziro Y. A new extragenic suppressor of cya mutation. Mutant cyclic AMP receptor protein with an increased affinity for cyclic AMP. J Biochem. 1978 Jun;83(6):1615–1623. doi: 10.1093/oxfordjournals.jbchem.a132073. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Tillier F., Monod J. Catabolite modulator factor: a possible mediator of catabolite repression in bacteria. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3476–3479. doi: 10.1073/pnas.73.10.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzan M., Favre R., Gallay E., Caro L. Genetical and structural analysis of a group of lambda ilv and lambda rho transducing phages. Mol Gen Genet. 1981;182(3):462–470. doi: 10.1007/BF00293936. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Kodaira R., Neidhardt F. C. Regulation of lac operon expression: reappraisal of the theory of catabolite repression. J Bacteriol. 1978 Dec;136(3):947–954. doi: 10.1128/jb.136.3.947-954.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne P. K., Rosen O. M. Cyclic 3':5'-adenosine monophosphate in Escherichia coli during transient and catabolite repression. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1436–1440. doi: 10.1073/pnas.71.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. K., Bloom R. W., Epstein W. Catabolite and transient repression in Escherichia coli do not require enzyme I of the phosphotransferase system. J Bacteriol. 1979 Apr;138(1):275–279. doi: 10.1128/jb.138.1.275-279.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]