Abstract
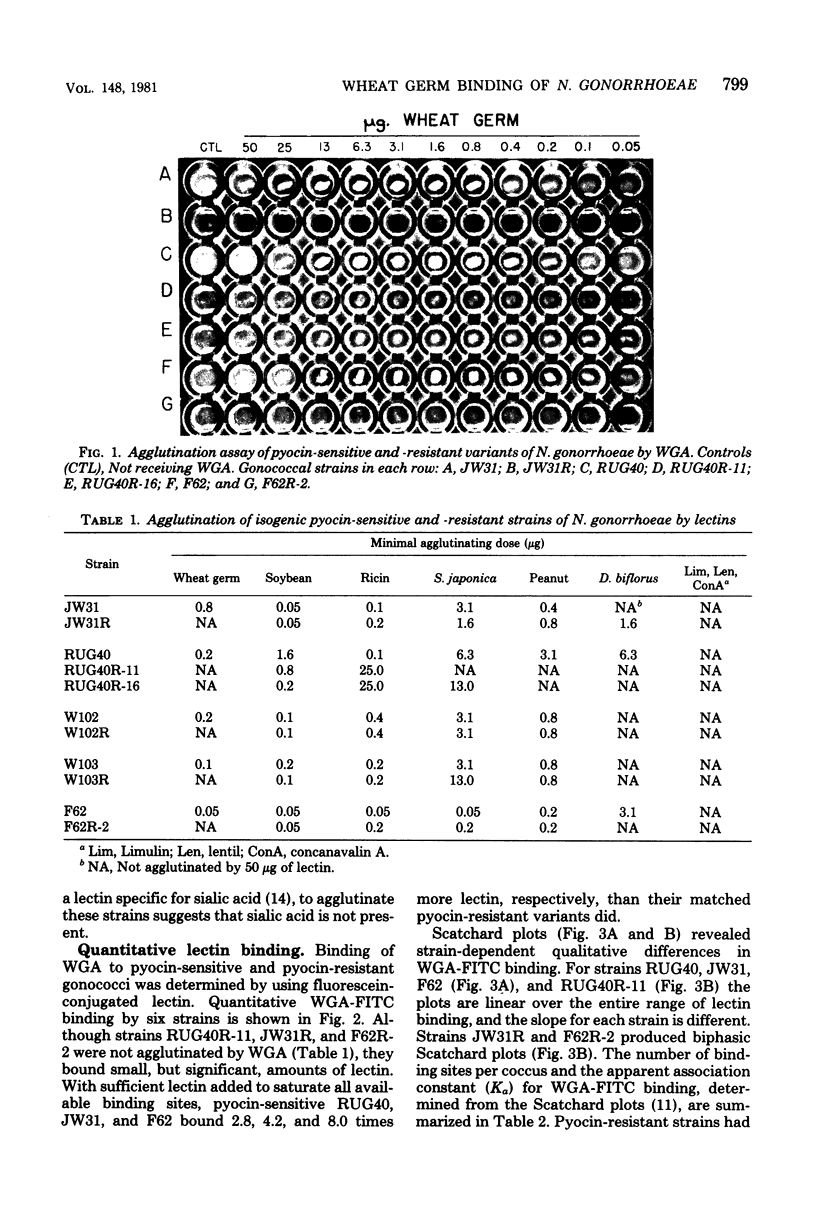
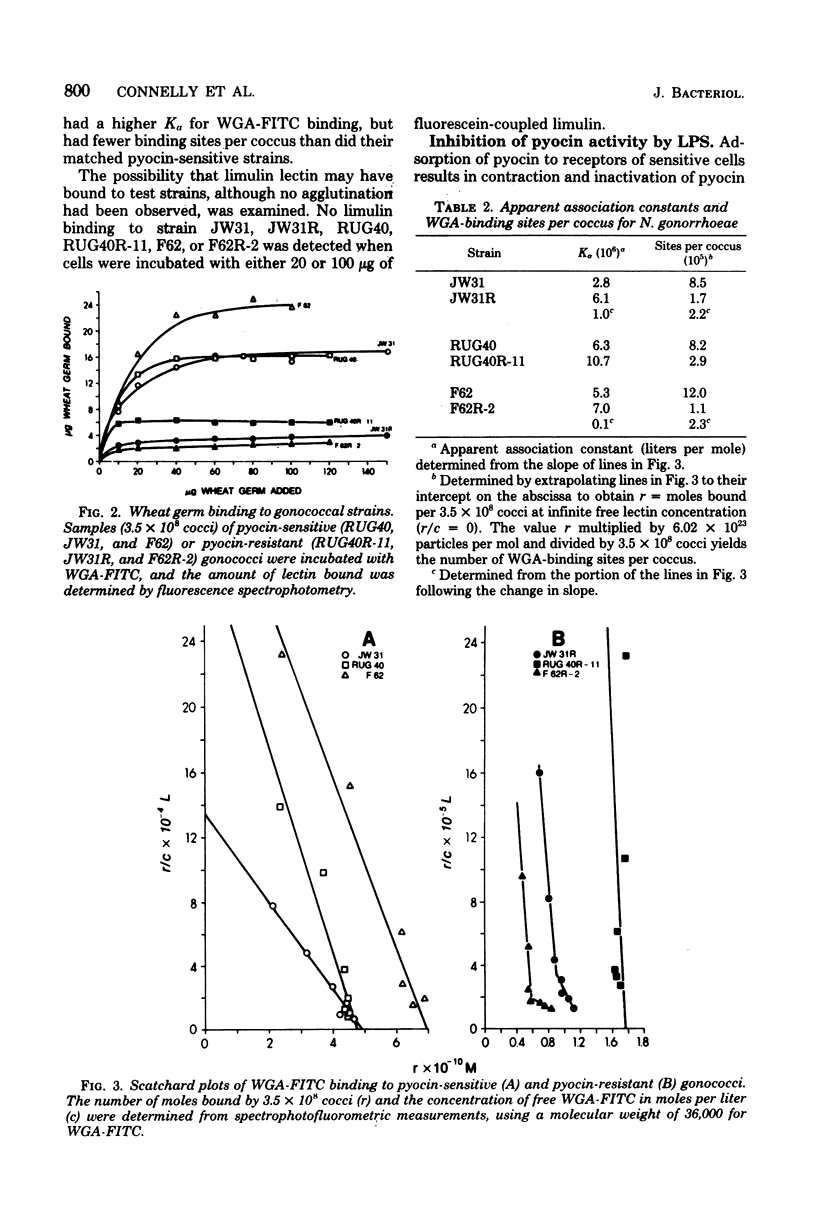
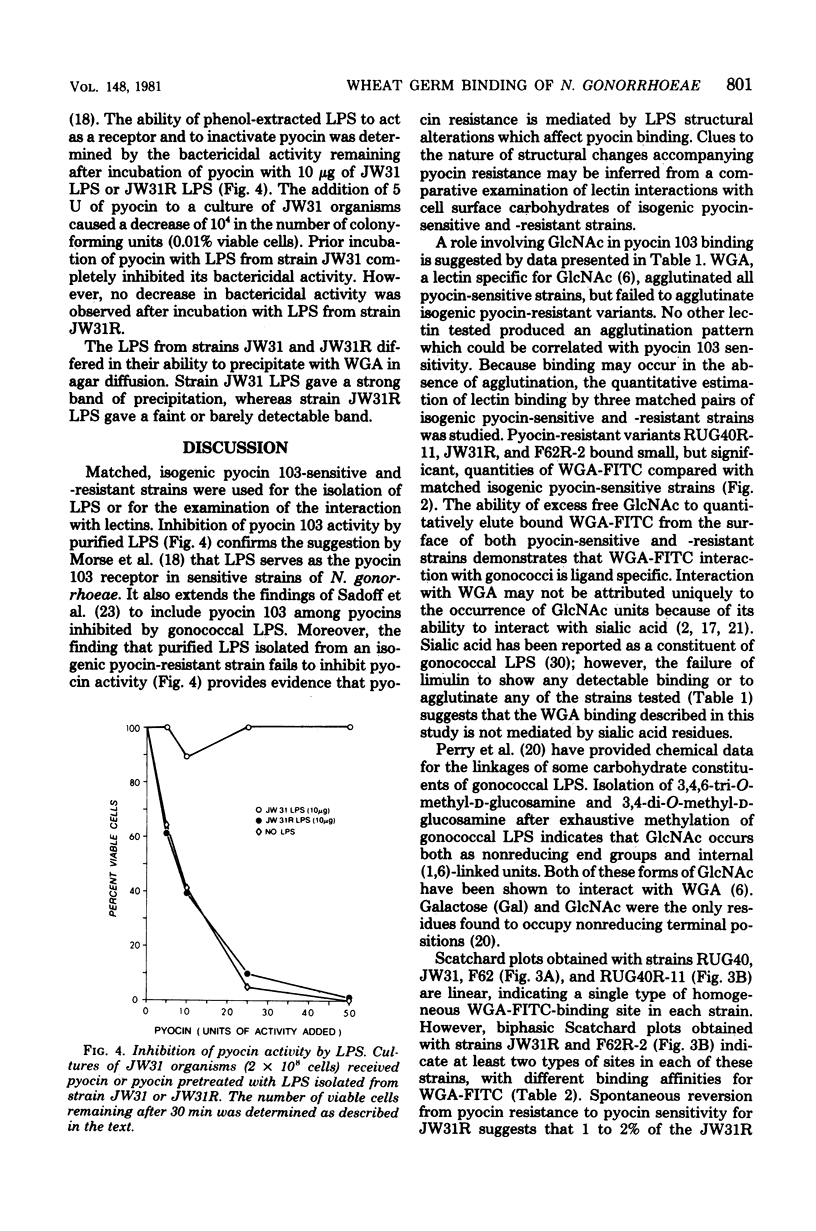
Strains of Neisseria gonorrhoeae were treated with pyocin 611 131 (pyocin 103) from Pseudomonas aeruginosa PA103, and isogenic resistant variants were isolated. The interaction of pyocin-sensitive and isogenic pyocin-resistant strains with wheat germ agglutinin (WGA) agglutinated all pyocin-sensitive, but not pyocin-resistant, strains. Binding of WGA to three pyocin-sensitive strains and their isogenic pyocin-resistant variants was examined quantitatively by using fluorescein-conjugated lectin. Pyocin-resistant strains maximally bound one-third to one-eighth the quantity of WGA bound by isogenic-sensitive strains. Linear Scatchard plots revealed homogeneous WGA-binding sites on three pyocin-sensitive and one pyocin-resistant strains. Biphasic Scatchard plots, obtained with two pyocin-resistant strains, show that WGA-binding sites in these strains are heterogeneous. The number of WGA-binding sites for pyocin-sensitive organisms ranged from 8 x 10(5) to 1 x 10(6) sites per coccus and from 1 x 10(5) to 3 x 10(5) sites per coccus for pyocin-resistant strains. The apparent association constant for WGA binding to pyocin-sensitive strains ranged from 3 x 10(6) to 6 x 10(6) liters/mol and from 6 x 10(6) to 1 x 10(7) liters/mol for pyocin-resistant strains. Gonococcal lipopolysaccharide was shown to serve as the pyocin 103 receptor by inhibition of pyocin activity. Lipopolysaccharide from a pyocin 103-resistant strain was not able to inhibit pyocin 103 activity. Pyocin 103 resistance was correlated with a structural alteration involving N-acetylglucosamine residues in gonococcal lipopolysaccharide. Based on interactions with wheat germ, soybean, and ricin lectins, a model of lipopolysaccharide structure in N. gonorrhoeae is presented.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. Z., Connelly M. C., Apicella M. A. Interaction of lectins with Neisseria gonorrhoeae. Can J Microbiol. 1980 Apr;26(4):468–474. doi: 10.1139/m80-078. [DOI] [PubMed] [Google Scholar]
- Bhavanandan V. P., Katlic A. W. The interaction of wheat germ agglutinin with sialoglycoproteins. The role of sialic acid. J Biol Chem. 1979 May 25;254(10):4000–4008. [PubMed] [Google Scholar]
- Dyke J., Berk R. S. Growth inhibition and pyocin receptor properties of endotoxin from Pseudomonas aeruginosa. Proc Soc Exp Biol Med. 1974 Apr;145(4):1405–1408. doi: 10.3181/00379727-145-38023. [DOI] [PubMed] [Google Scholar]
- Frasch C. E. Role of lipopolysaccharide in wheat germ agglutinin-mediated agglutination of Neisseria meningitidis and Neisseria gonorrhoeae. J Clin Microbiol. 1980 Oct;12(4):498–501. doi: 10.1128/jcm.12.4.498-501.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Knutson D. W., Kijlstra A., Van Es L. A. Association and dissociation of aggregated IgG from rat peritoneal macrophages. J Exp Med. 1977 May 1;145(5):1368–1381. doi: 10.1084/jem.145.5.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Young F. E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974 Jul;28(1):70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R., Sharon N., Mirelman D. Interaction of wheat-germ agglutinin with bacterial cells and cell-wall polymers. Eur J Biochem. 1975 Jun 16;55(1):257–262. doi: 10.1111/j.1432-1033.1975.tb02158.x. [DOI] [PubMed] [Google Scholar]
- Maget-Dana R., Veh R. W., Sander M., Roche A. C., Schauer R., Monsigny M. Specificities of limulin and wheat-germ agglutinin towards some derivatives of GM3 gangliosides. Eur J Biochem. 1981;114(1):11–16. doi: 10.1111/j.1432-1033.1981.tb06164.x. [DOI] [PubMed] [Google Scholar]
- Monsigny M., Roche A. C., Sene C., Maget-Dana R., Delmotte F. Sugar-lectin interactions: how does wheat-germ agglutinin bind sialoglycoconjugates? Eur J Biochem. 1980 Feb;104(1):147–153. doi: 10.1111/j.1432-1033.1980.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Vaughan P., Johnson D., Iglewski B. H. Inhibition of Neisseria gonorrhoeae by a bacteriocin from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Aug;10(2):354–362. doi: 10.1128/aac.10.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M. B., Daoust V. The lipopolysaccharides of Neisseria gonorrhoeae colony types 1 and 4. Can J Biochem. 1975 May;53(5):623–629. doi: 10.1139/o75-084. [DOI] [PubMed] [Google Scholar]
- Peters B. P., Ebisu S., Goldstein I. J., Flashner M. Interaction of wheat germ agglutinin with sialic acid. Biochemistry. 1979 Nov 27;18(24):5505–5511. doi: 10.1021/bi00591a038. [DOI] [PubMed] [Google Scholar]
- Schaefer R. L., Keller K. F., Doyle R. J. Lectins in diagnostic microbiology: use of wheat germ agglutinin for laboratory identification of Neisseria gonorrhoeae. J Clin Microbiol. 1979 Nov;10(5):669–672. doi: 10.1128/jcm.10.5.669-672.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidberry H. D., Sadoff J. C. Pyocin sensitivity of Neisseria gonorrhoeae and its feasibility as an epidemiological tool. Infect Immun. 1977 Feb;15(2):628–637. doi: 10.1128/iai.15.2.628-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead A., Main J. S., Ward M. E., Watt P. J. Studies on lipopolysaccharides isolated from strains of Neisseria gonorrhoeae. J Gen Microbiol. 1975 May;88(1):123–131. doi: 10.1099/00221287-88-1-123. [DOI] [PubMed] [Google Scholar]
- Stein D. C., Hebeler B. H., Young F. E. Effect of environment on sensitivity of Neisseria gonorrhoeae to Pseudomonas aeruginosa bacteriocins. Infect Immun. 1980 Aug;29(2):507–511. doi: 10.1128/iai.29.2.507-511.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE L. A., KELLOGG D. S., Jr NEISSERIA GONORRHOEAE IDENTIFICATION IN DIRECT SMEARS BY A FLUORESCENT ANTIBODY-COUNTERSTAIN METHOD. Appl Microbiol. 1965 Mar;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman G. M., Caird J. D. Composition of the lipopolysaccharide of Neisseria gonorrhoeae. Infect Immun. 1977 May;16(2):550–556. doi: 10.1128/iai.16.2.550-556.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



