Abstract
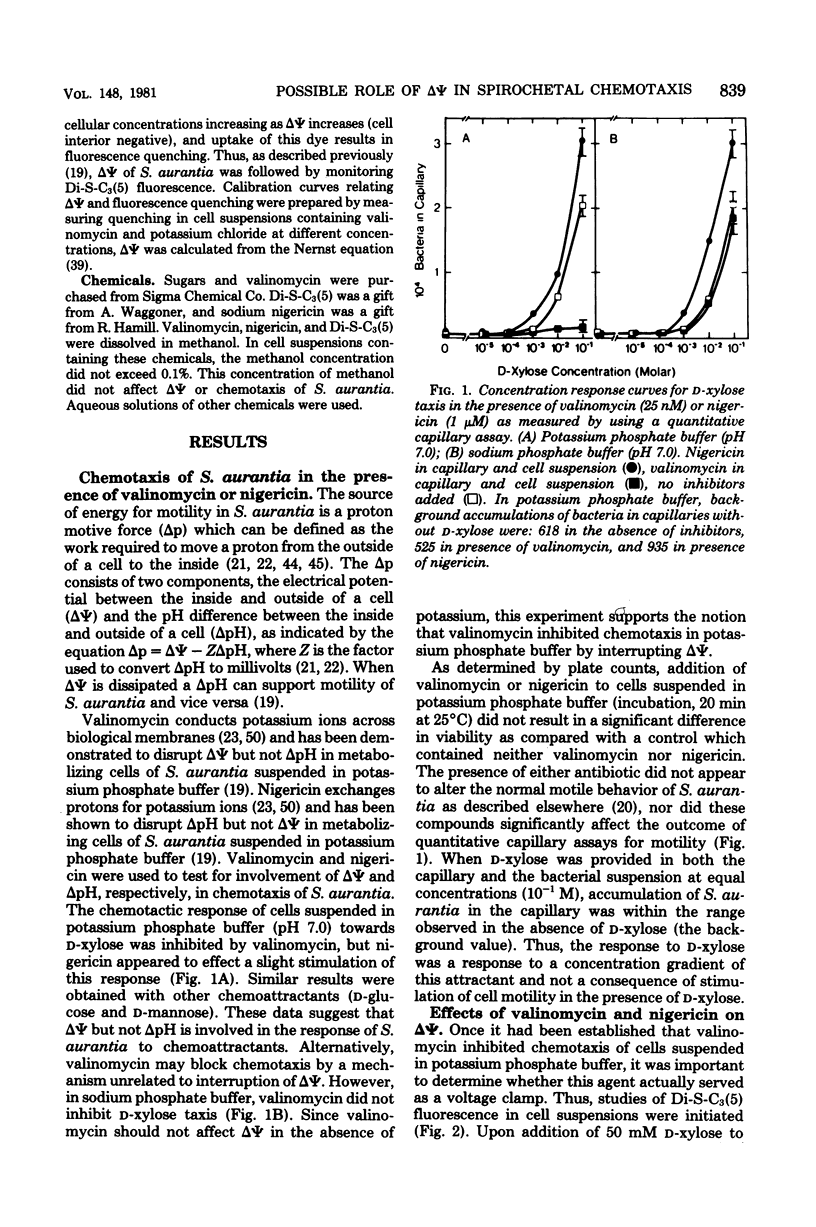
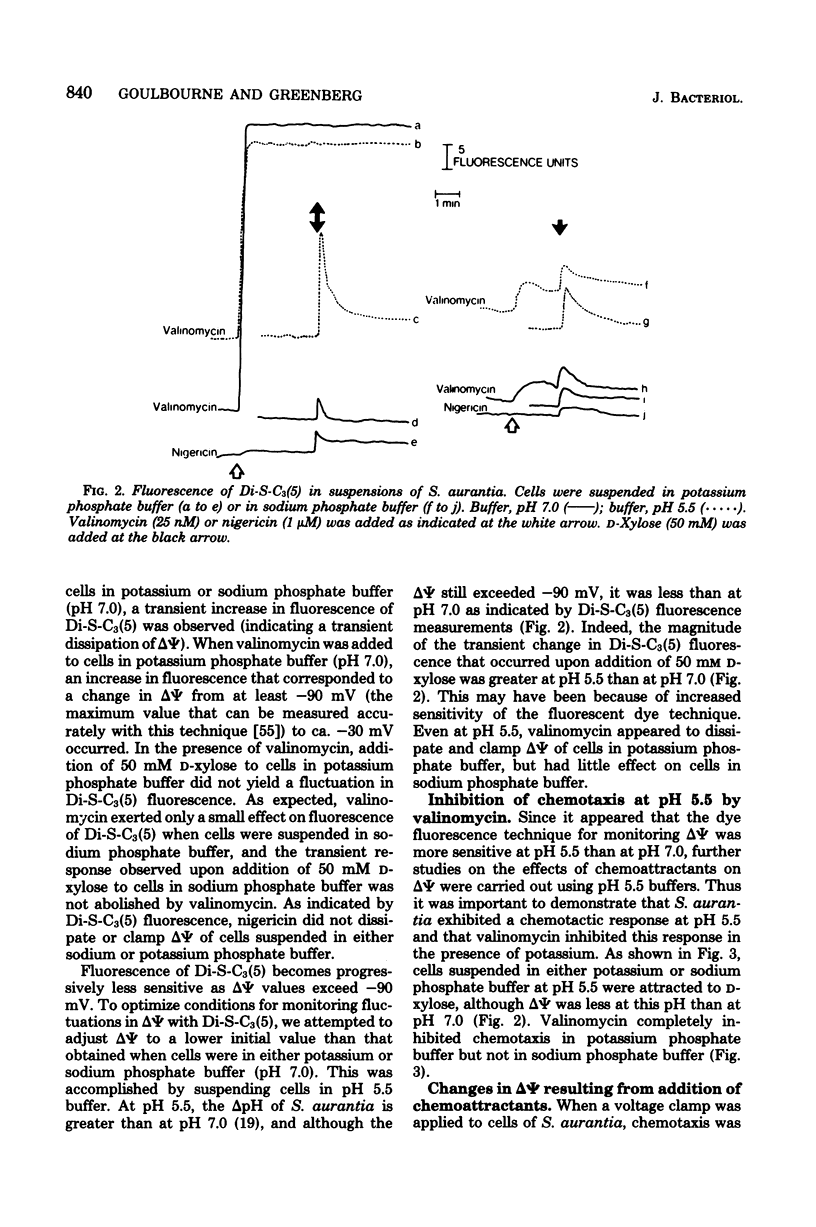
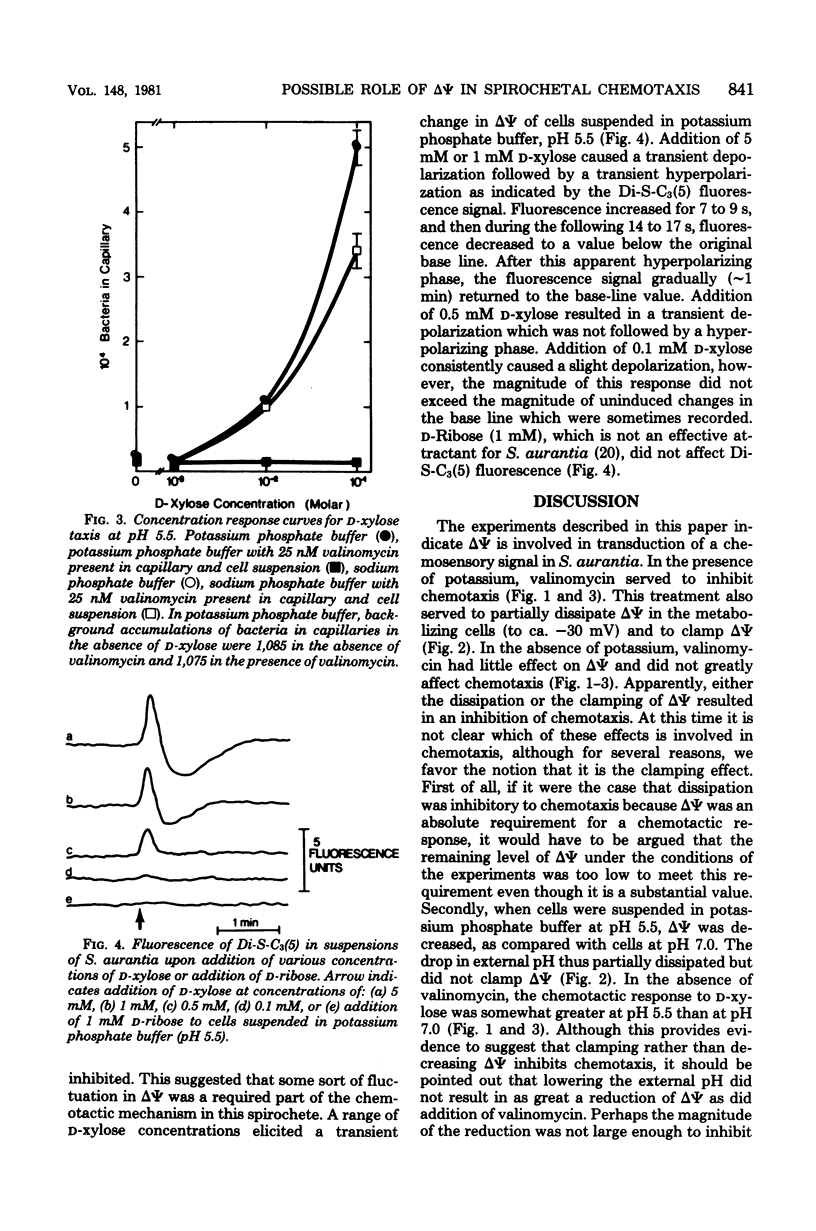
The effects of valinomycin and nigericin on sugar chemotaxis in Spirochaeta aurantia were investigated by using a quantitative capillary assay, and the fluorescent cation, 3,3'-dipropyl-2,2'-thiodicarbocyanine iodide was used as a probe to study effects of chemoattractants on membrane potential. Addition of a chemoattractant, D-xylose, to cells in either potassium or sodium phosphate buffer resulted in a transient membrane depolarization. In the presence of valinomycin, the membrane potential of cells in potassium phosphate buffer was reduced, and the transient membrane depolarization that resulted from the addition of D-xylose was eliminated. Although there was no detectable effect of valinomycin on motility, D-xylose taxis of cells in potassium phosphate buffer was completely inhibited by valinomycin. In sodium phosphate buffer, valinomycin had little effect on membrane potential or D-xylose taxis. Nigericin is known to dissipate the transmembrane pH gradient of S. aurantia in potassium phosphate buffer. This compound did not dissipate the membrane potential or the transient membrane depolarization observed upon addition of D-xylose to cells in either potassium or sodium phosphate buffer. Nigericin did not inhibit D-xylose taxis in either potassium or sodium phosphate buffer. This study indicates that the membrane potential but not the transmembrane pH gradient of S. aurantia is somehow involved in chemosensory signal transduction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Adler J. The behavior of bacteria: on the mechanism of sensory transduction in bacterial chemotaxis. Johns Hopkins Med J. 1979 Apr;144(4):121–126. [PubMed] [Google Scholar]
- Armitage J. P., Evans M. C. Chemotactically induced increase in the membrane potential of spheroplasts of Rhodopseudomonas sphaeroides. FEBS Lett. 1980 Mar 24;112(1):5–9. doi: 10.1016/0014-5793(80)80113-x. [DOI] [PubMed] [Google Scholar]
- Armitage J. P., Evans M. C. Membrane potential changes during chemotaxis of Rhodopseudomonas sphaeroides. FEBS Lett. 1979 Jun 1;102(1):143–146. doi: 10.1016/0014-5793(79)80946-1. [DOI] [PubMed] [Google Scholar]
- Berg H. C. How spirochetes may swim. J Theor Biol. 1976 Feb;56(2):269–273. doi: 10.1016/s0022-5193(76)80074-4. [DOI] [PubMed] [Google Scholar]
- Bharier M. A., Eiserling F. A., Rittenberg S. C. Eletron microscopic observations on the structure of Treponema zuelzerae and its axial filaments. J Bacteriol. 1971 Jan;105(1):413–421. doi: 10.1128/jb.105.1.413-421.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharier M., Allis D. Purification and characterization of axial filaments from Treponema phagedenis biotype reiterii (the Reiter treponeme). J Bacteriol. 1974 Dec;120(3):1434–1442. doi: 10.1128/jb.120.3.1434-1442.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. A., Hobson A. C., Adler J. Involvement of cyclic GMP in intracellular signaling in the chemotactic response of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3879–3883. doi: 10.1073/pnas.77.7.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznak J. A., Canale-Parola E. Morphology and physiology of Spirochaeta aurantia strains isolated from aquatic habitats. Arch Microbiol. 1975 Sep 30;105(1):1–12. doi: 10.1007/BF00447104. [DOI] [PubMed] [Google Scholar]
- Bromley D. B., Charon N. W. Axial filament involvement in the motility of Leptospira interrogans. J Bacteriol. 1979 Mar;137(3):1406–1412. doi: 10.1128/jb.137.3.1406-1412.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canale-Parola E. Motility and chemotaxis of spirochetes. Annu Rev Microbiol. 1978;32:69–99. doi: 10.1146/annurev.mi.32.100178.000441. [DOI] [PubMed] [Google Scholar]
- Canale-Parola E. Physiology and evolution of spirochetes. Bacteriol Rev. 1977 Mar;41(1):181–204. doi: 10.1128/br.41.1.181-204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Glagolev A. N., Skulachev V. P. The proton pump is a molecular engine of motile bacteria. Nature. 1978 Mar 16;272(5650):280–282. doi: 10.1038/272280a0. [DOI] [PubMed] [Google Scholar]
- Goulbourne E. A., Jr, Greenberg E. P. Relationship between proton motive force and motility in Spirochaeta aurantia. J Bacteriol. 1980 Sep;143(3):1450–1457. doi: 10.1128/jb.143.3.1450-1457.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg E. P., Canale-Parola E. Chemotaxis in Spirochaeta aurantia. J Bacteriol. 1977 Apr;130(1):485–494. doi: 10.1128/jb.130.1.485-494.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Altendorf K. H., Hirata H. Probing membrane transport mechanisms with inophores. Ann N Y Acad Sci. 1974 May 10;235(0):149–160. doi: 10.1111/j.1749-6632.1974.tb43264.x. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Engström P. Parallel pathways for transduction of chemotactic signals in Escherichia coli. Nature. 1980 Jan 3;283(5742):98–100. doi: 10.1038/283098a0. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Canale-Parola E. Fine structure of Spirochaeta stenostrepta, a free-living, anaerobic spirochete. J Bacteriol. 1968 Sep;96(3):822–835. doi: 10.1128/jb.96.3.822-835.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougen K. H., Birch-Andersen A. Electron microscopy of endoflagella and microtubules in Treponema reiter. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(1):37–50. doi: 10.1111/j.1699-0463.1971.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Jackson S., Black S. H. Ultrastructure of Treponema pallidum Nichols following lysis by physical and chemical methods. II. Axial filaments. Arch Mikrobiol. 1971;76(4):325–340. doi: 10.1007/BF00408529. [DOI] [PubMed] [Google Scholar]
- Joseph R., Canale-Parola E. Axial fibrils of anaerobic spirochetes: ultrastructure and chemical characteristics. Arch Mikrobiol. 1972;81(2):146–168. doi: 10.1007/BF00412325. [DOI] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. Proton chemical potential, proton electrical potential and bacterial motility. J Mol Biol. 1980 Apr 15;138(3):599–614. doi: 10.1016/s0022-2836(80)80019-2. [DOI] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. The steady-state counterclockwise/clockwise ratio of bacterial flagellar motors is regulated by protonmotive force. J Mol Biol. 1980 Apr 15;138(3):563–597. doi: 10.1016/s0022-2836(80)80018-0. [DOI] [PubMed] [Google Scholar]
- Kihara M., Macnab R. M. Cytoplasmic pH mediates pH taxis and weak-acid repellent taxis of bacteria. J Bacteriol. 1981 Mar;145(3):1209–1221. doi: 10.1128/jb.145.3.1209-1221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C., Chang S. Y., Satow Y., Houten J. V., Hansma H. Genetic dissection of behavior in paramecium. Science. 1975 May 30;188(4191):898–904. [PubMed] [Google Scholar]
- LISTGARTEN M. A., SOCRANSKY S. S. ELECTRON MICROSCOPY OF AXIAL FIBRILS, OUTER ENVELOPE, AND CELL DIVISION OF CERTAIN ORAL SPIROCHETES. J Bacteriol. 1964 Oct;88:1087–1103. doi: 10.1128/jb.88.4.1087-1103.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. H., Adler J., Gargus J. J., Hogg R. W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Macnab R. M. Bacterial motility and chemotaxis: the molecular biology of a behavioral system. CRC Crit Rev Biochem. 1978;5(4):291–341. doi: 10.3109/10409237809177145. [DOI] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P. M., Berg H. C. Energetics of flagellar rotation in bacteria. J Mol Biol. 1980 Apr 15;138(3):541–561. doi: 10.1016/s0022-2836(80)80017-9. [DOI] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P., Berg H. C., Harold F. M., Van der Drift C. A protonmotive force drives bacterial flagella. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsura S., Shioi J., Imae Y. Motility in Bacillus subtilis driven by an artificial protonmotive force. FEBS Lett. 1977 Oct 15;82(2):187–190. doi: 10.1016/0014-5793(77)80581-4. [DOI] [PubMed] [Google Scholar]
- Matsuura S., Shioi J. I., Imae Y., Iida S. Characterization of the Bacillus subtilis motile system driven by an artificially created proton motive force. J Bacteriol. 1979 Oct;140(1):28–36. doi: 10.1128/jb.140.1.28-36.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. B., Koshland D. E., Jr Protonmotive force and bacterial sensing. J Bacteriol. 1980 Jan;141(1):26–32. doi: 10.1128/jb.141.1.26-32.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. B., Koshland D. E., Jr Sensory electrophysiology of bacteria: relationship of the membrane potential to motility and chemotaxis in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4752–4756. doi: 10.1073/pnas.74.11.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Nauman R. K., Holt S. C., Cox C. D. Purification, ultrastructure, and composition of axial filaments from Leptospira. J Bacteriol. 1969 Apr;98(1):264–280. doi: 10.1128/jb.98.1.264-280.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W. Calcium ion regulates chemotactic behaviour in bacteria. Nature. 1977 Nov 3;270(5632):66–67. doi: 10.1038/270066a0. [DOI] [PubMed] [Google Scholar]
- Paster B. J., Canale-Parola E. Involvement of periplasmic fibrils in motility of spirochetes. J Bacteriol. 1980 Jan;141(1):359–364. doi: 10.1128/jb.141.1.359-364.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Repaske D. R., Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol. 1981 Mar;145(3):1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway H. F. Source of energy for gliding motility in Flexibacter polymorphus: effects of metabolic and respiratory inhibitors on gliding movement. J Bacteriol. 1977 Aug;131(2):544–556. doi: 10.1128/jb.131.2.544-556.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi J. I., Imae Y., Oosawa F. Protonmotive force and motility of Bacillus subtilis. J Bacteriol. 1978 Mar;133(3):1083–1088. doi: 10.1128/jb.133.3.1083-1088.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Adler J. Change in membrane potential during bacterial chemotaxis. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4387–4391. doi: 10.1073/pnas.73.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner A. Optical probes of membrane potential. J Membr Biol. 1976 Jun 30;27(4):317–334. doi: 10.1007/BF01869143. [DOI] [PubMed] [Google Scholar]


