Abstract
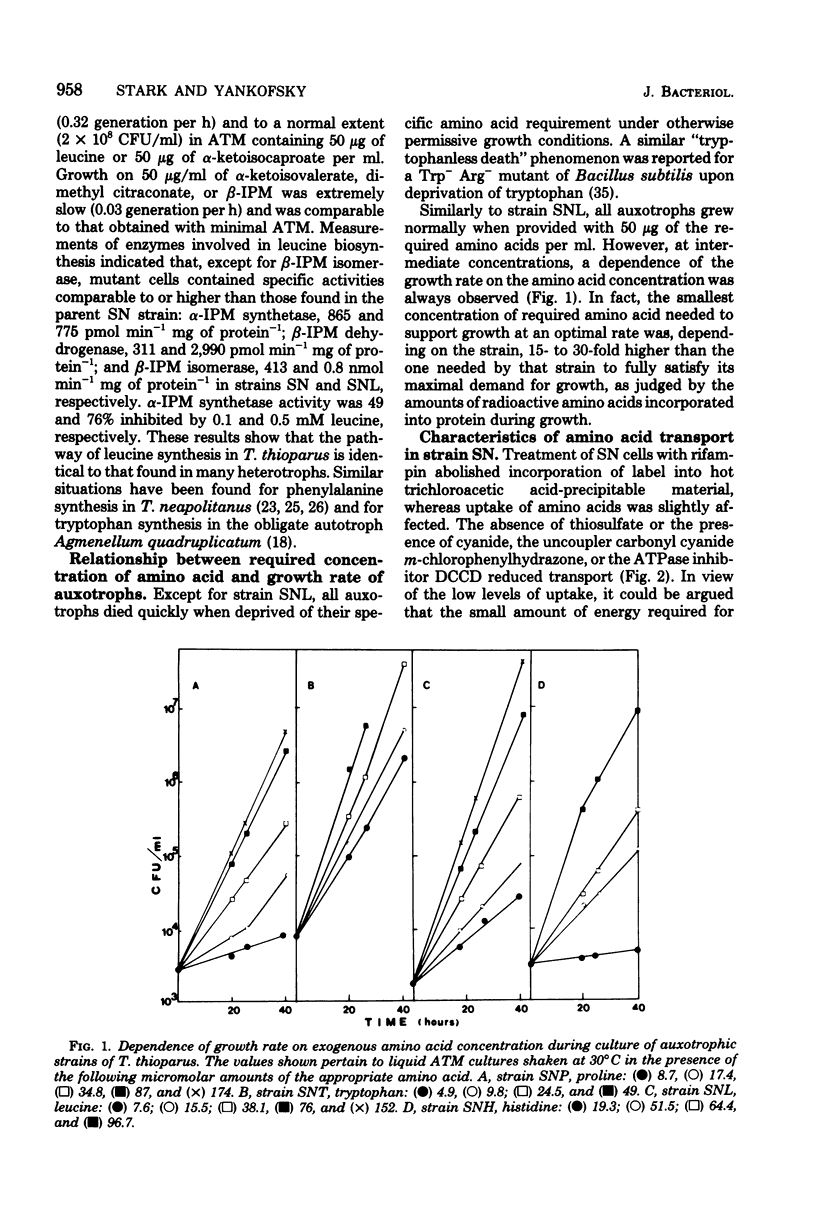
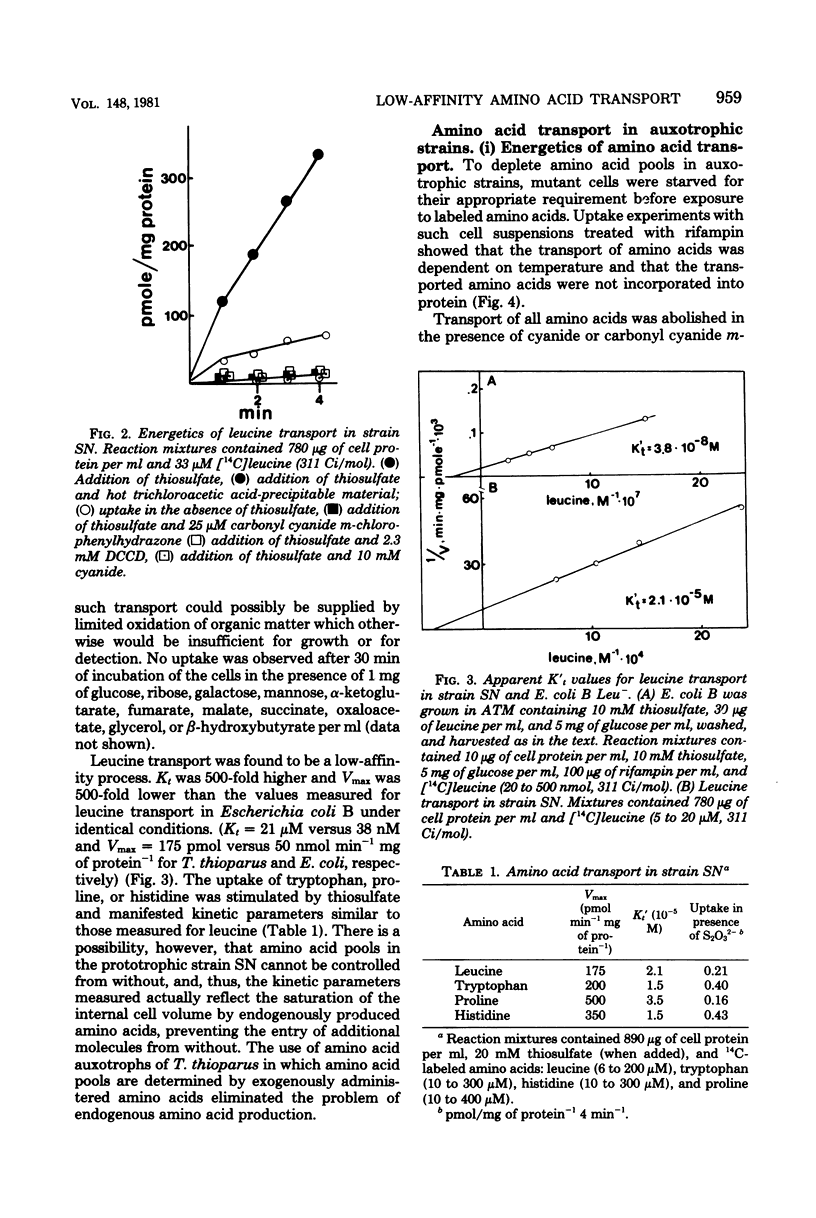
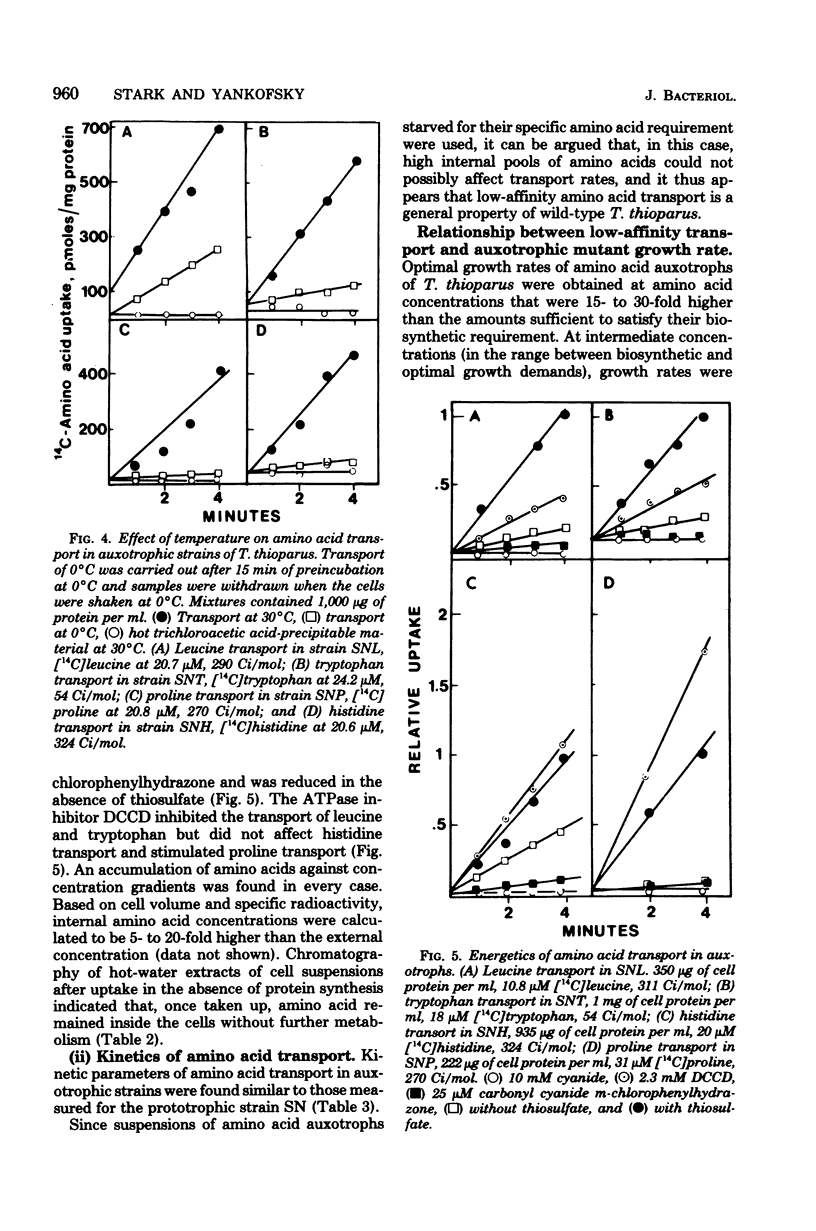
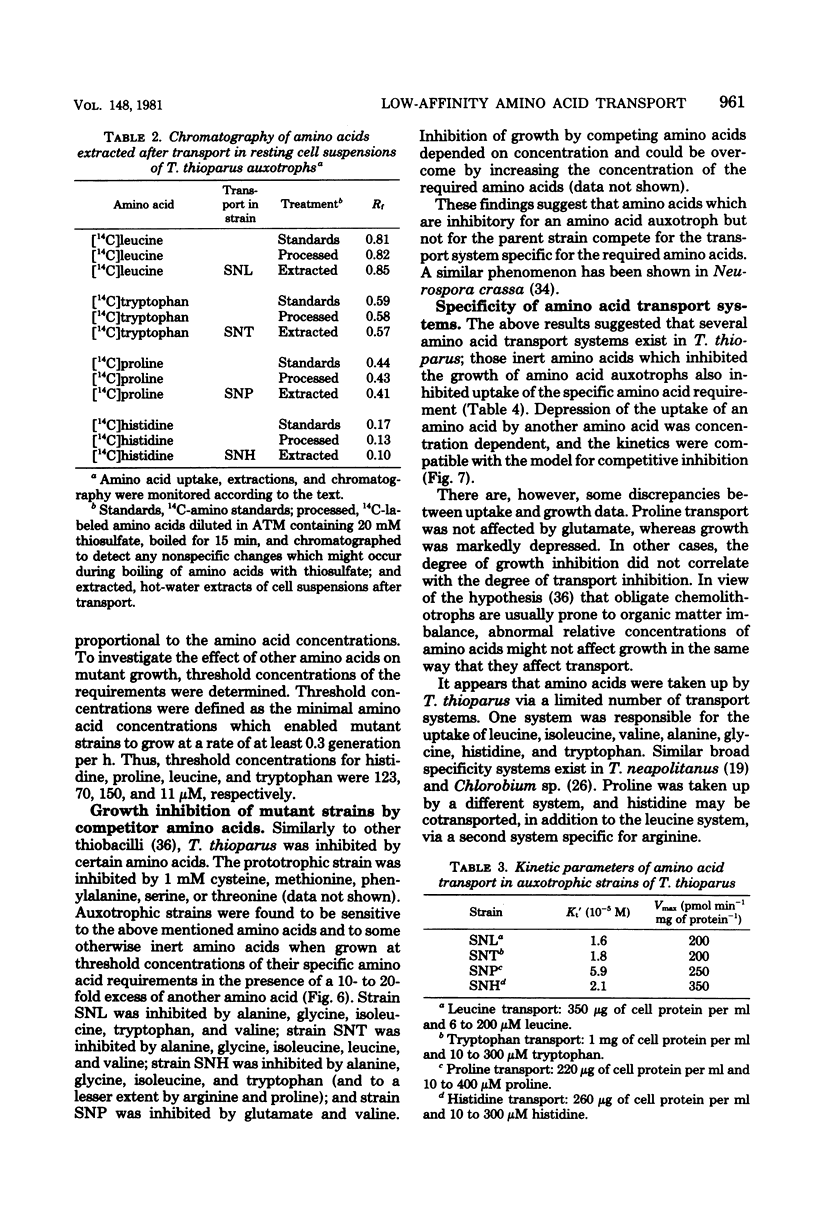
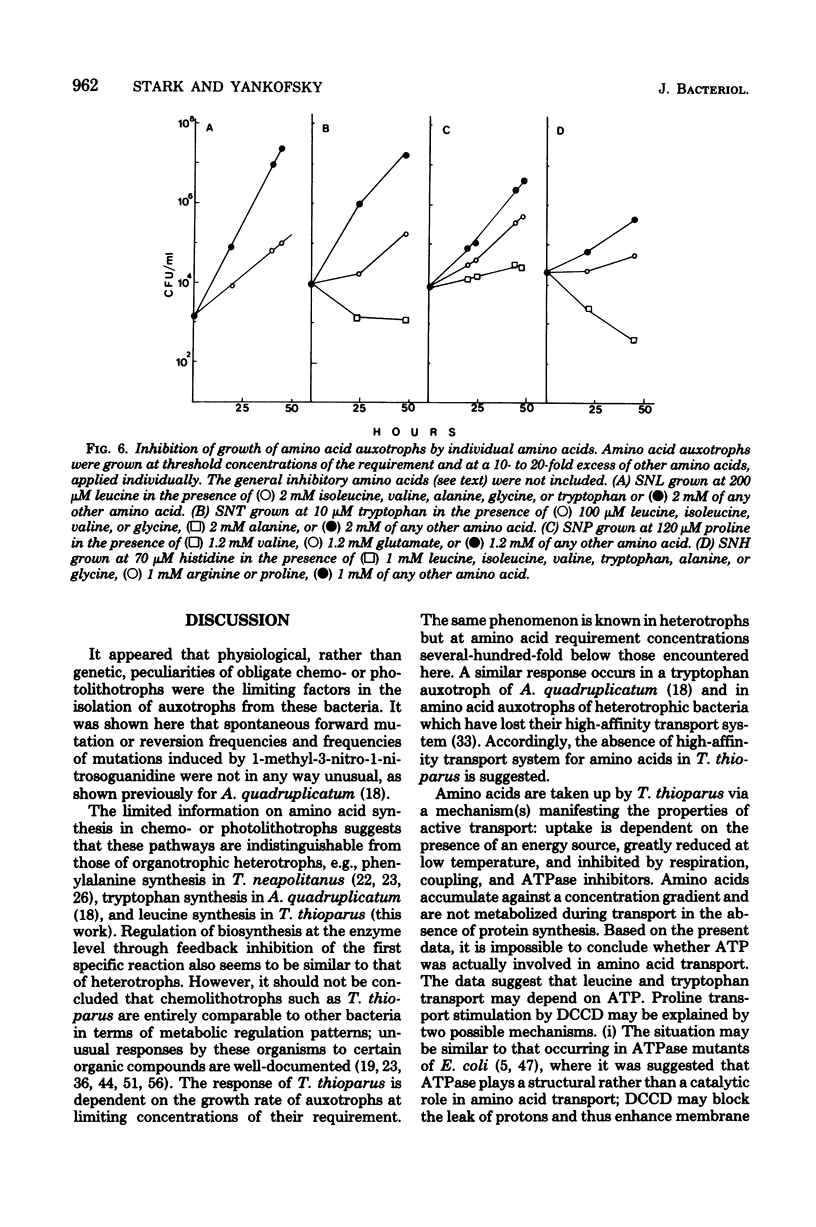
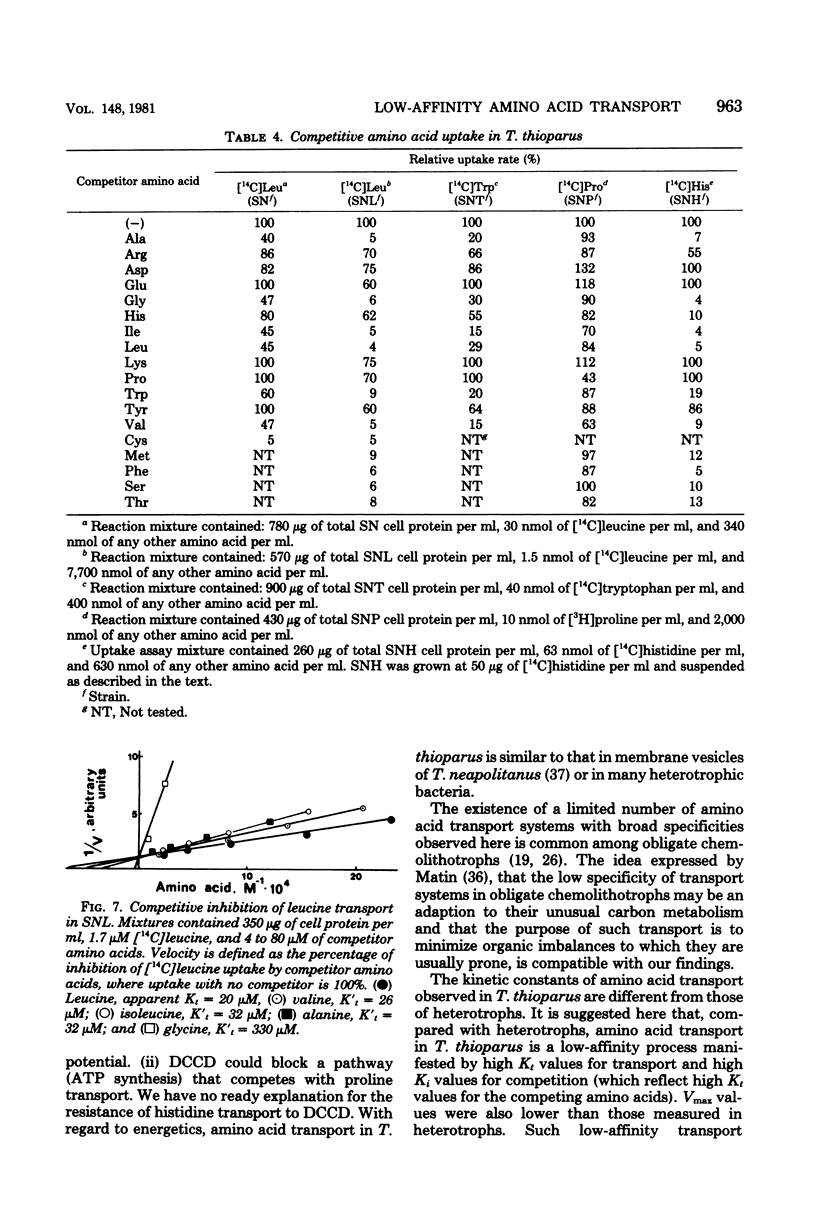
A method for the isolation of amino acid auxotrophs of Thiobacillus thioparus is described. Characterization of a leucine auxotroph indicated that leucine biosynthesis in T. thioparus was not different from that of heterotrophic bacteria. T. thioparus cells accumulated amino acids via an active mechanism. Kt values of amino acid transport were between 15 and 330 microM, and Vmax values were 200 to 350 pmol min-1 mg of protein-1. Amino acid transport was carried out by a limited number of systems, each responsible for the uptake of several amino acids. Amino acid auxotrophs of T. thioparus exhibited transport and growth properties similar to those of transport-deficient mutants of heterotrophs which lost the high-affinity, but retained the low-affinity, amino acid transport systems.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Roth J. R. Histidine and aromatic permeases of Salmonella typhimurim. J Bacteriol. 1968 Nov;96(5):1742–1749. doi: 10.1128/jb.96.5.1742-1749.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayling P. D., Bridgeland E. S. Methionine transport in wild-type and transport-defective mutants of Salmonella typhimurium. J Gen Microbiol. 1972 Nov;73(1):127–141. doi: 10.1099/00221287-73-1-127. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley J. A., Brierley C. L. Urea as a nitrogen source for thiobacilli. J Bacteriol. 1968 Aug;96(2):573–573. doi: 10.1128/jb.96.2.573-.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Maintenance and exchange of the aromatic amino acid pool in Escherichia coli. J Bacteriol. 1971 Apr;106(1):70–81. doi: 10.1128/jb.106.1.70-81.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis T. F., Rosenfeld H. J., Maas W. K. Mutant of Escherichia coli K-12 defective in the transport of basic amino acids. J Bacteriol. 1973 Nov;116(2):619–626. doi: 10.1128/jb.116.2.619-626.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C. L., Miller D. L., Rodwell V. W. Metabolism of basic amino acids in Pseudomonas putida. Transport of lysine, ornithine, and arginine. J Biol Chem. 1972 Apr 25;247(8):2283–2288. [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Klopotowski T., Iaccarino M. Multiplicity of isoleucine, leucine, and valine transport systems in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):382–392. doi: 10.1128/jb.117.2.382-392.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guroff G., Bromwell K., Abramowitz A. Mode of action of p-chlorophenylalanine on Pseudomonas species (11299a). Arch Biochem Biophys. 1969 May;131(2):543–550. doi: 10.1016/0003-9861(69)90428-7. [DOI] [PubMed] [Google Scholar]
- Halpern Y. S. Genetics of amino acid transport in bacteria. Annu Rev Genet. 1974;8:103–133. doi: 10.1146/annurev.ge.08.120174.000535. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Pierson D., Kane J. F., Van Baalen C., Jensen R. A. Documentation of auxotrophic mutation in blue-green bacteria: characterization of a tryptophan auxotroph in Agmenellum quadruplicatum. J Bacteriol. 1972 Jul;111(1):112–118. doi: 10.1128/jb.111.1.112-118.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. L., Vishniac W. Growth Inhibition in Thiobacillus neapolitanus by Histidine, Methionine, Phenylalanine, and Threonine. J Bacteriol. 1970 Dec;104(3):1145–1150. doi: 10.1128/jb.104.3.1145-1150.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSEL D., LUBIN M. Transport of proline in Escherichia coli. Biochim Biophys Acta. 1962 Feb 12;57:32–43. doi: 10.1016/0006-3002(62)91074-0. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Proline transport by Pseudomonas aeruginosa. Biochim Biophys Acta. 1969;193(2):444–455. doi: 10.1016/0005-2736(69)90203-x. [DOI] [PubMed] [Google Scholar]
- Kelly D. P. Autotrophy: concepts of lithotrophic bacteria and their organic metabolism. Annu Rev Microbiol. 1971;25:177–210. doi: 10.1146/annurev.mi.25.100171.001141. [DOI] [PubMed] [Google Scholar]
- Kelly D. P. Influence of amino acids and organic antimetabolites on growth and biosynthesis of the chemoautotroph Thiobacillus neapolitanus strain C. Arch Mikrobiol. 1967 Feb 20;56(2):91–105. doi: 10.1007/BF00408761. [DOI] [PubMed] [Google Scholar]
- Kelly D. P. Regulation of chemoautotrophic metabolism. 3. DAHP synthetase in Thiobacillus neapolitanus. Arch Mikrobiol. 1969;69(4):360–369. doi: 10.1007/BF00408576. [DOI] [PubMed] [Google Scholar]
- Kelly D. P. Regulation of chemoautotrophic metabolism. I. Toxicity of phenylalanine to thiobacilli. Arch Mikrobiol. 1969;69(4):330–342. doi: 10.1007/BF00408574. [DOI] [PubMed] [Google Scholar]
- Kelly D. P. Regulation of chemoautotrophic metabolism. II. Competition between amino acids for incorporation into Thiobacillus. Arch Mikrobiol. 1969;69(4):343–359. [PubMed] [Google Scholar]
- Kelly D. P. The incorporation of acetate by the chemoautotroph Thiobacillus neapolitanus strain C. Arch Mikrobiol. 1967;58(2):99–116. doi: 10.1007/BF00406671. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUBIN M., KESSEL D. H., BUDREAU A., GROSS J. D. The isolation of bacterial mutants defective in amino acid transport. Biochim Biophys Acta. 1960 Aug 26;42:535–538. doi: 10.1016/0006-3002(60)90836-2. [DOI] [PubMed] [Google Scholar]
- Lee M., Robbins J. C., Oxender D. L. Transport properties of merodiploids covering the dagA locus in Escherichia coli K-12. J Bacteriol. 1975 Jun;122(3):1001–1005. doi: 10.1128/jb.122.3.1001-1005.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. L., Rédei G. P., Gowans C. S. A phylogenetic comparison of mutation spectra. Mol Gen Genet. 1967;100(1):77–83. doi: 10.1007/BF00425777. [DOI] [PubMed] [Google Scholar]
- Lombardi F. J., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. 8. The transport of amino acids by membranes prepared from Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):7844–7857. [PubMed] [Google Scholar]
- Magill J. M., Magill C. W. Correlation of growth inhibition patterns to nucleoside transport models in Neurospora crassa. J Bacteriol. 1973 Nov;116(2):1071–1074. doi: 10.1128/jb.116.2.1071-1074.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerfeld I., Barlati S., Ciferri O. Tryptophanless death in Bacillus subtilis. J Bacteriol. 1970 Feb;101(2):350–354. doi: 10.1128/jb.101.2.350-354.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Konings W. N., Kuenen J. G., Emmens M. Active transport of amino acids by membrane vesicles of Thiobacillus neapolitanus. J Gen Microbiol. 1974 Aug;83(2):311–318. doi: 10.1099/00221287-83-2-311. [DOI] [PubMed] [Google Scholar]
- Matin A. Organic nutrition of chemolithotrophic bacteria. Annu Rev Microbiol. 1978;32:433–468. doi: 10.1146/annurev.mi.32.100178.002245. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Rodwell V. W. Metabolism of basic amino acids in Pseudomonas putida. Properties of the inducible lysine transport system. J Biol Chem. 1971 Mar 25;246(6):1765–1771. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- Prasad R., Kalra V. K., Brodie A. F. Active transport of glutamine and glutamic acid in membrane vesicles from Mycobacterium phlei. Biochem Biophys Res Commun. 1975 Mar 3;63(1):50–56. doi: 10.1016/s0006-291x(75)80009-x. [DOI] [PubMed] [Google Scholar]
- Rahmanian M., Claus D. R., Oxender D. L. Multiplicity of leucine transport systems in Escherichia coli K-12. J Bacteriol. 1973 Dec;116(3):1258–1266. doi: 10.1128/jb.116.3.1258-1266.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli. II. Purification and properties of an arginine-specific binding protein. J Biol Chem. 1973 Feb 25;248(4):1211–1218. [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli. J Biol Chem. 1971 Jun 10;246(11):3653–3662. [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli: properties of canavanine-resistant mutants. J Bacteriol. 1973 Nov;116(2):627–635. doi: 10.1128/jb.116.2.627-635.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P. Restoration of active transport in an Mg2+-adenosine triphosphatase-deficient mutant of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1124–1129. doi: 10.1128/jb.116.3.1124-1129.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., White D. C., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. IX. The kinetics and specificity of amino acid transport in Staphylococcus aureus membrane vesicles. J Biol Chem. 1972 Dec 10;247(23):7452–7458. [PubMed] [Google Scholar]
- Smith A. J., Hoare D. S. Specialist phototrophs, lithotrophs, and methylotrophs: a unity among a diversity of procaryotes? Bacteriol Rev. 1977 Jun;41(2):419–448. doi: 10.1128/br.41.2.419-448.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. F., Hoare D. S., Hoare S. L. Thiobacillus denitrificans as an obligate chemolithotroph. Isolation and growth studies. Arch Mikrobiol. 1971;78(3):193–204. doi: 10.1007/BF00424893. [DOI] [PubMed] [Google Scholar]
- Tristram H., Neale S. The activity and specificity of the proline permease in wild-type and analogue-resistant strains of Escherichia coli. J Gen Microbiol. 1968 Jan;50(1):121–137. doi: 10.1099/00221287-50-1-121. [DOI] [PubMed] [Google Scholar]
- Weiner J. H., Furlong C. E., Heppel L. A. A binding protein for L-glutamine and its relation to active transport in E. coli. Arch Biochem Biophys. 1971 Feb;142(2):715–717. doi: 10.1016/0003-9861(71)90538-8. [DOI] [PubMed] [Google Scholar]


