Abstract
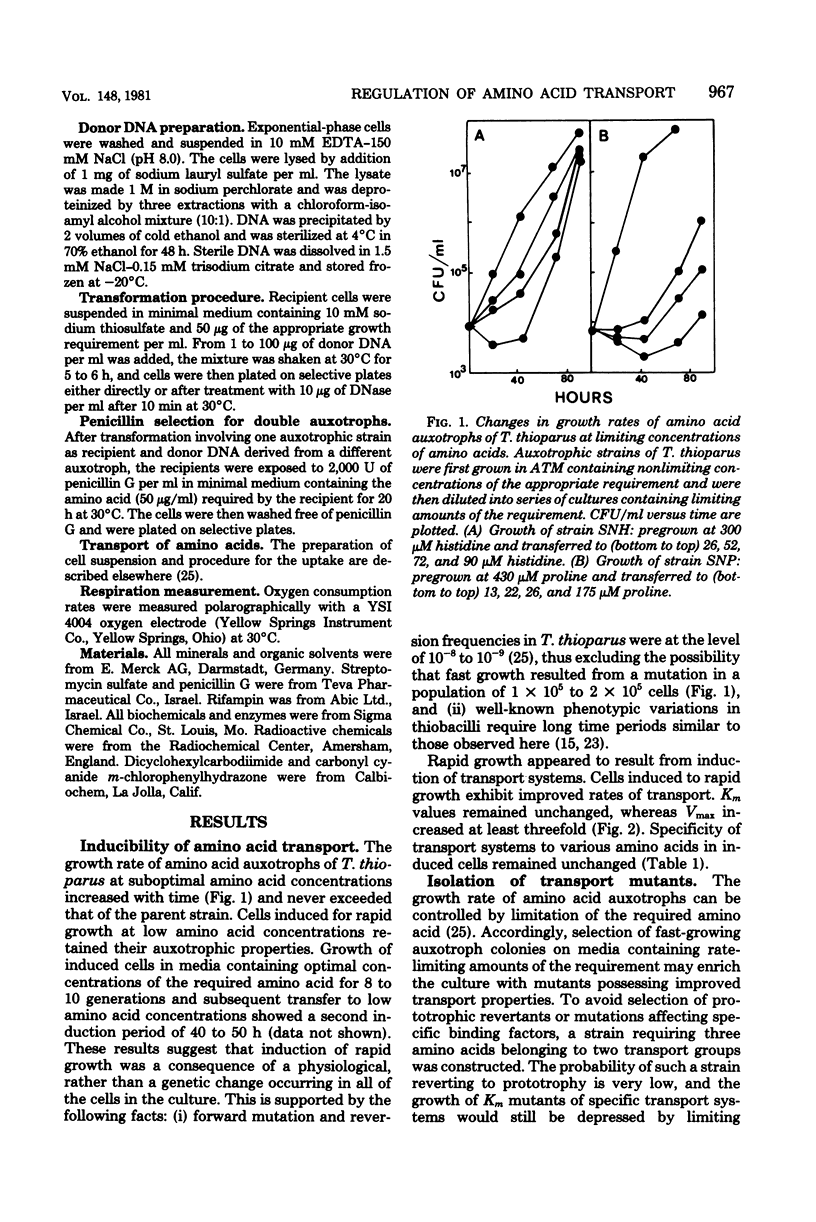
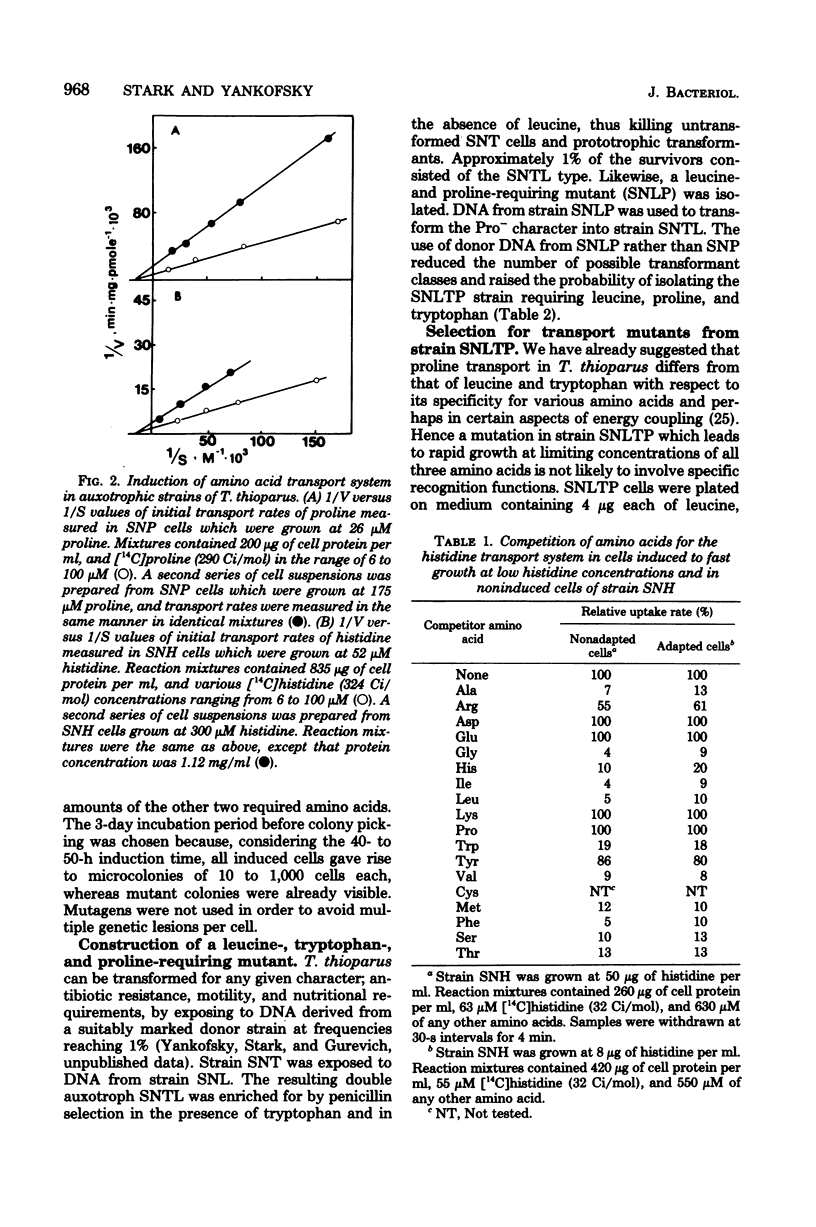
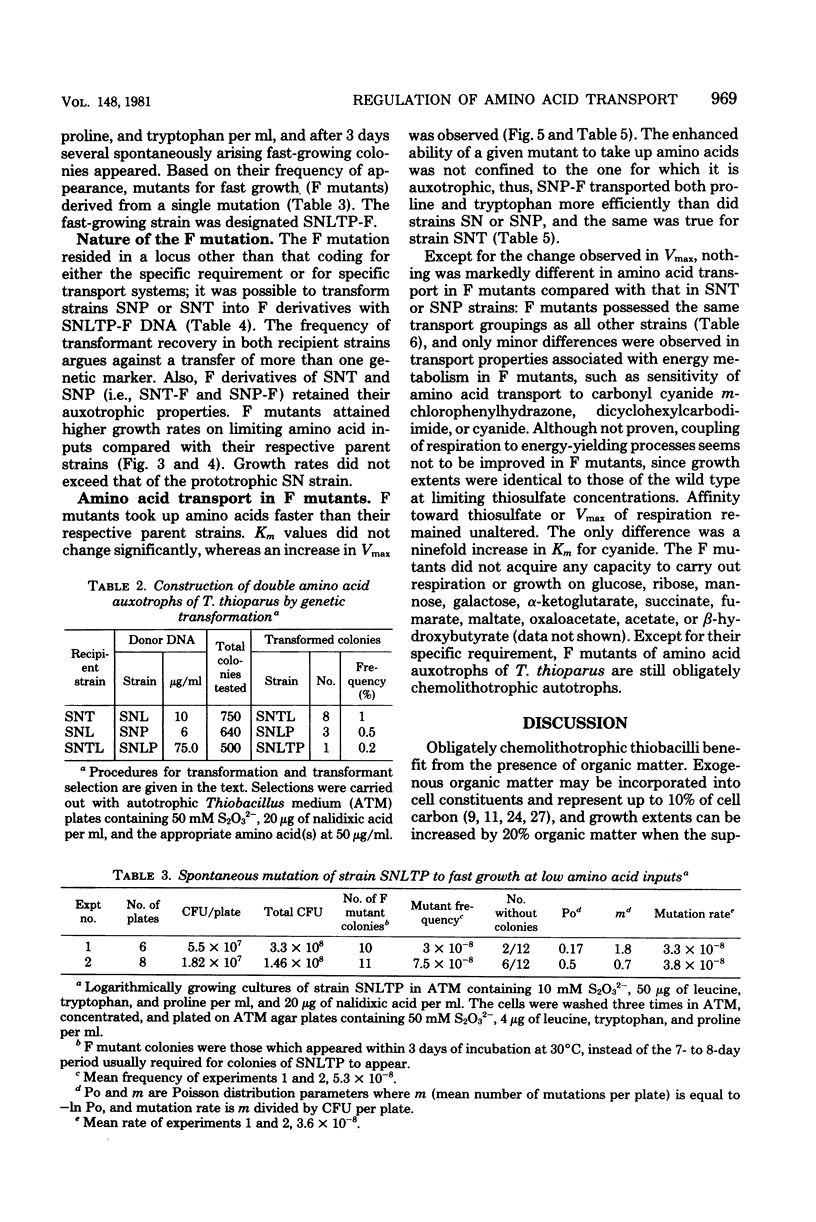
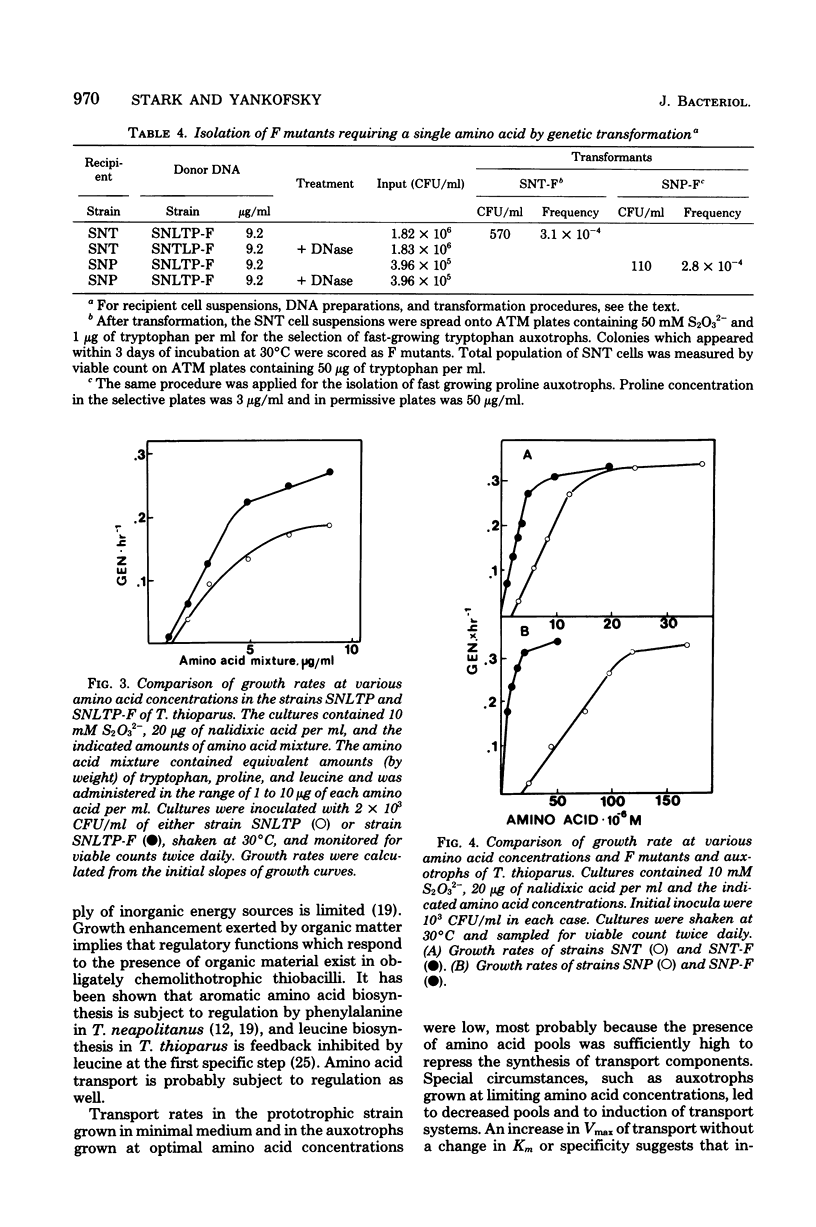
Amino acid transport in amino acid auxotrophs of Thiobacillus thioparus was enhanced during growth on rate-limiting amino acid concentration. A pleiotropic mutation enhanced general amino acid transport as manifested by higher values of Vmax of amino acid transport. Affinity constants remained unaltered. Mutants with enhanced transport properties did not show changes in oxidation of thiosulfate, did not oxidize various organic compounds, and did not increase the heterotrophic potential of T. thioparus. The mutations for enhanced transport caused increased synthesis of amino acid transport system components. A method for genetic transformation of T. thioparus is described.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURROUS S. E., DEMOSS R. D. STUDIES ON TRYPTOPHAN PERMEASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 6;73:623–637. doi: 10.1016/0006-3002(63)90332-9. [DOI] [PubMed] [Google Scholar]
- Cole J. S., 3rd, Aleem M. I. Oxidative phosphorylation in Thiobacillus novellus. Biochem Biophys Res Commun. 1970 Feb 20;38(4):736–743. doi: 10.1016/0006-291x(70)90643-1. [DOI] [PubMed] [Google Scholar]
- Guroff G., Bromwell K., Abramowitz A. Mode of action of p-chlorophenylalanine on Pseudomonas species (11299a). Arch Biochem Biophys. 1969 May;131(2):543–550. doi: 10.1016/0003-9861(69)90428-7. [DOI] [PubMed] [Google Scholar]
- Halpern Y. S., Even-Shoshan A. Properties of the glutamate transport system in Escherichia coli. J Bacteriol. 1967 Mar;93(3):1009–1016. doi: 10.1128/jb.93.3.1009-1016.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Lupo M. Glutamate transport in wild-type and mutant strains of Escherichia coli. J Bacteriol. 1965 Nov;90(5):1288–1295. doi: 10.1128/jb.90.5.1288-1295.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempfling W. P., Vishniac W. Oxidative phosphorylation in extracts of thiobacillus X. Biochem Z. 1965 Aug 6;342(3):272–287. [PubMed] [Google Scholar]
- KESSEL D., LUBIN M. Transport of proline in Escherichia coli. Biochim Biophys Acta. 1962 Feb 12;57:32–43. doi: 10.1016/0006-3002(62)91074-0. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Proline transport by Pseudomonas aeruginosa. Biochim Biophys Acta. 1969;193(2):444–455. doi: 10.1016/0005-2736(69)90203-x. [DOI] [PubMed] [Google Scholar]
- Kelly D. P. Regulation of chemoautotrophic metabolism. 3. DAHP synthetase in Thiobacillus neapolitanus. Arch Mikrobiol. 1969;69(4):360–369. doi: 10.1007/BF00408576. [DOI] [PubMed] [Google Scholar]
- Kelly D. P. Regulation of chemoautotrophic metabolism. I. Toxicity of phenylalanine to thiobacilli. Arch Mikrobiol. 1969;69(4):330–342. doi: 10.1007/BF00408574. [DOI] [PubMed] [Google Scholar]
- Kelly D. P. Regulation of chemoautotrophic metabolism. II. Competition between amino acids for incorporation into Thiobacillus. Arch Mikrobiol. 1969;69(4):343–359. [PubMed] [Google Scholar]
- Kelly D. P. The incorporation of acetate by the chemoautotroph Thiobacillus neapolitanus strain C. Arch Mikrobiol. 1967;58(2):99–116. doi: 10.1007/BF00406671. [DOI] [PubMed] [Google Scholar]
- Krajewska-Grynkiewicz K., Walczak W., Klopotowski T. Mutants of Salmonella typhimurium able to utilize D-histidine as a source of L-histidine. J Bacteriol. 1971 Jan;105(1):28–37. doi: 10.1128/jb.105.1.28-37.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUBIN M., KESSEL D. H., BUDREAU A., GROSS J. D. The isolation of bacterial mutants defective in amino acid transport. Biochim Biophys Acta. 1960 Aug 26;42:535–538. doi: 10.1016/0006-3002(60)90836-2. [DOI] [PubMed] [Google Scholar]
- London J., Rittenberg S. C. Effects of organic matter on the growth of Thiobacillus intermedius. J Bacteriol. 1966 Mar;91(3):1062–1069. doi: 10.1128/jb.91.3.1062-1069.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. Genetic analysis of glutamate transport and glutamate decarboxylase in Escherichia coli. J Bacteriol. 1967 Apr;93(4):1409–1415. doi: 10.1128/jb.93.4.1409-1415.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. Genetic analysis of the glutamate permease in Escherichia coli K-12. J Bacteriol. 1969 Mar;97(3):1118–1128. doi: 10.1128/jb.97.3.1118-1128.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Konings W. N., Kuenen J. G., Emmens M. Active transport of amino acids by membrane vesicles of Thiobacillus neapolitanus. J Gen Microbiol. 1974 Aug;83(2):311–318. doi: 10.1099/00221287-83-2-311. [DOI] [PubMed] [Google Scholar]
- Matin A. Organic nutrition of chemolithotrophic bacteria. Annu Rev Microbiol. 1978;32:433–468. doi: 10.1146/annurev.mi.32.100178.002245. [DOI] [PubMed] [Google Scholar]
- Rahmanian M., Claus D. R., Oxender D. L. Multiplicity of leucine transport systems in Escherichia coli K-12. J Bacteriol. 1973 Dec;116(3):1258–1266. doi: 10.1128/jb.116.3.1258-1266.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli: properties of canavanine-resistant mutants. J Bacteriol. 1973 Nov;116(2):627–635. doi: 10.1128/jb.116.2.627-635.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafia F., Brinson K. R., Heinzman M. W., Brady J. M. Transition of chemolithotroph Ferrobacillus ferrooxidans to obligate organotrophy and metabolic capabilities of glucose-grown cells. J Bacteriol. 1972 Jul;111(1):56–65. doi: 10.1128/jb.111.1.56-65.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark A. A., Yankofsky S. A. Active transport of amino acids in Thiobacillus thioparus is a low-affinity process. J Bacteriol. 1981 Dec;148(3):956–965. doi: 10.1128/jb.148.3.956-965.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. F., Hoare D. S., Hoare S. L. Thiobacillus denitrificans as an obligate chemolithotroph. Isolation and growth studies. Arch Mikrobiol. 1971;78(3):193–204. doi: 10.1007/BF00424893. [DOI] [PubMed] [Google Scholar]


