Abstract
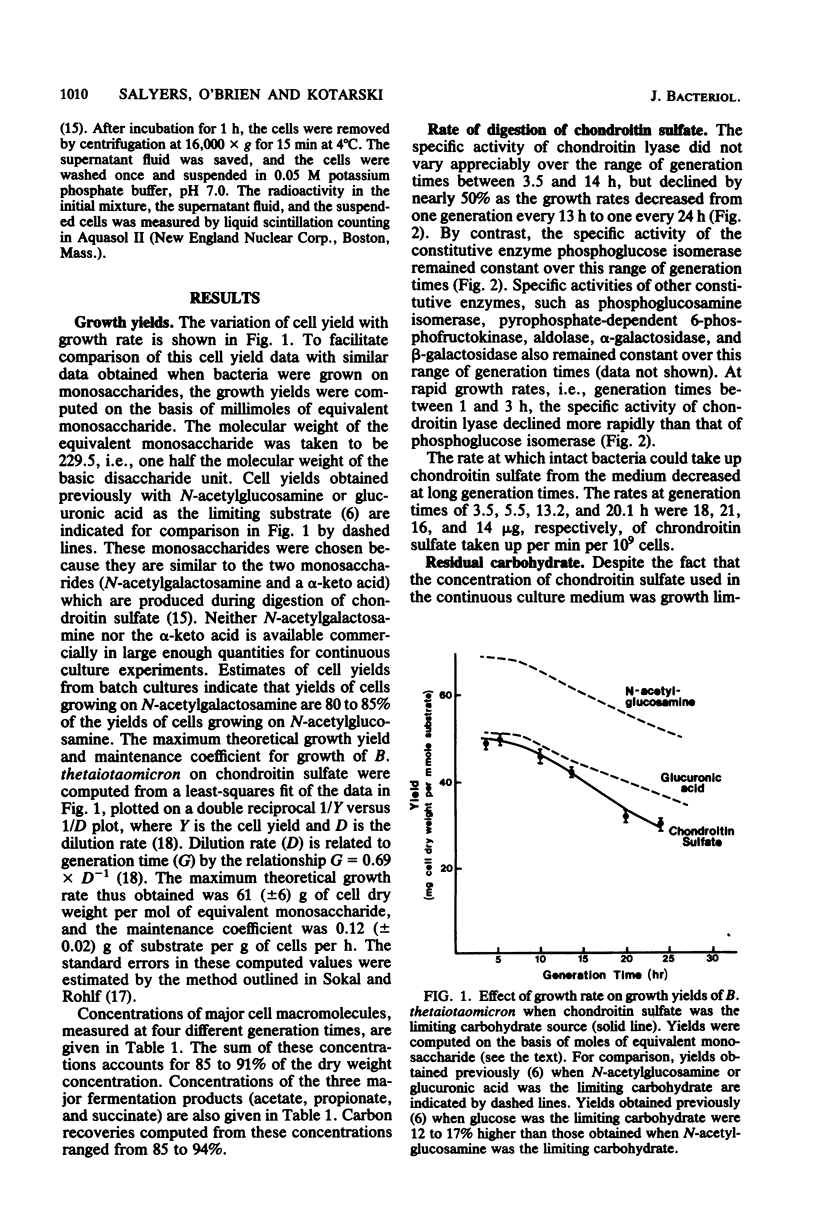
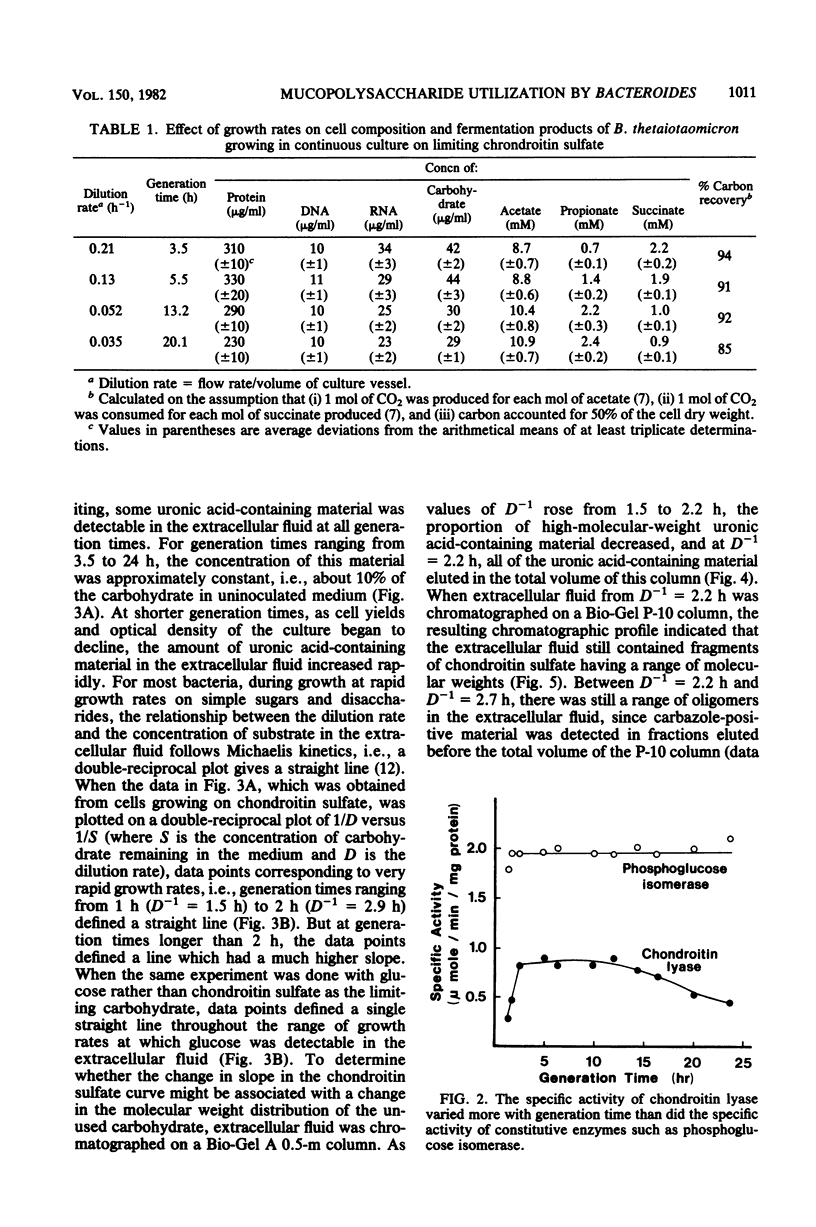
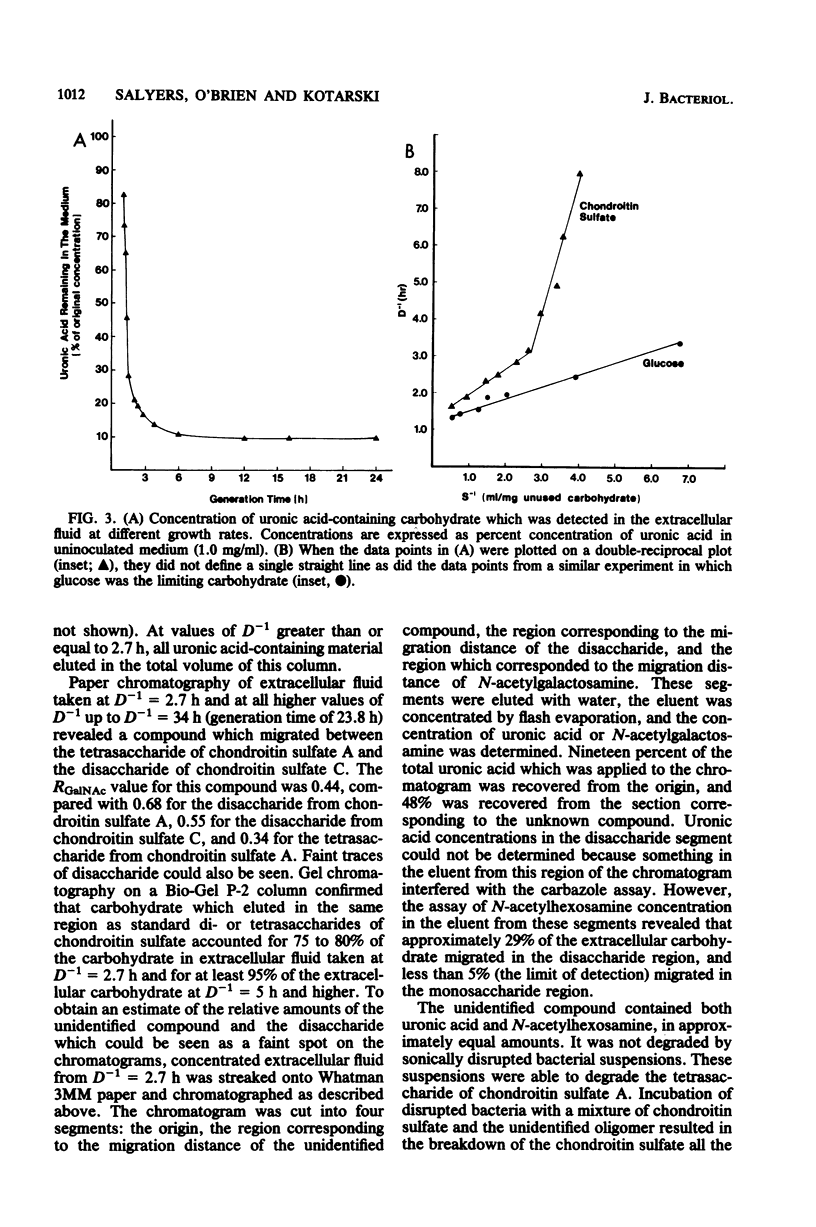
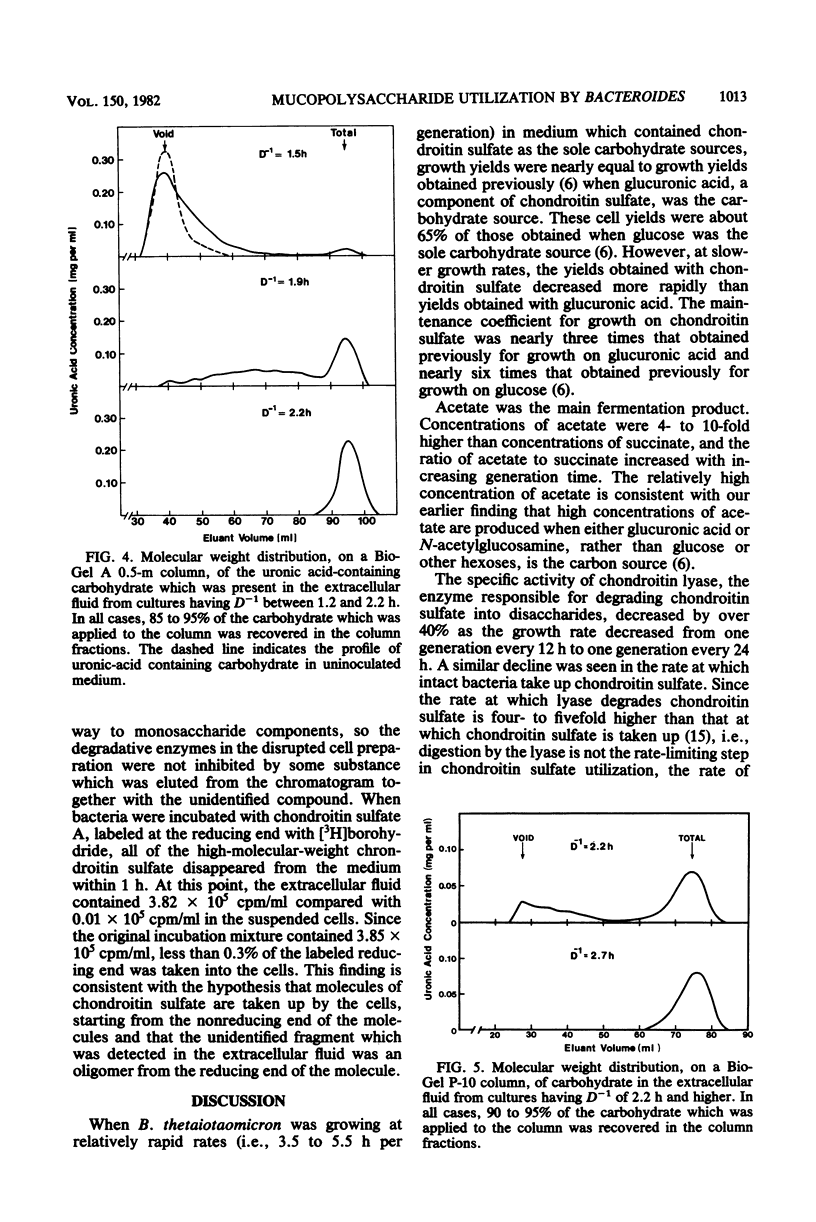
When Bacteroides thetaiotaomicron, an obligate anaerobe from the human colonic flora, was grown in continuous culture with the mucopolysaccharide chondroitin sulfate as the limiting source of carbohydrate, growth yields ranged from 48 g of cell dry weight per mol of equivalent monosaccharide at a growth rate of 3.5 h per generation to 32 g per mol at a growth rate of 24 h per generation. The theoretical maximum growth yield (61 g of cell dry weight per mol of equivalent monosaccharide) was comparable to that of 54 g per mol, which was obtained previously when glucuronic acid, a component of chondroitin sulfate, was the limiting carbohydrate (S. F. Kotarski and A. A. Salyers, J. Bacteriol. 146:853-860, 1981). However, the maintenance coefficient was three times higher when chondroitin sulfate was the substrate than when glucuronic acid was the substrate. The specific activity of chondroitin lyase (EC 4.2.2.4), an enzyme which cleaves chondroitin sulfate into disaccharides, declined by nearly 50% as growth rates decreased from 3.5 to 24 h per generation. By contrast, the specific activities of several glycolytic enzymes and disaccharidases remained constant over this range of growth rates. Although chondroitin sulfate was growth limiting, some carbohydrate was detectable in the extracellular fluid at all growth rates. At rapid growth rates (1 to 2 h per generation), this residual carbohydrate included fragments of chondroitin sulfate having a wide range of molecular weights. At slower growth rates (2 to 24 h per generation), the residual carbohydrate consisted mainly of a small fragment which migrated on paper chromatograms more slowly than the disaccharides produced by chondroitin lyase but faster than a tetrasaccharide. This small fragment may represent the reducing end of the chondroitin sulfate molecule.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. D. Microbial growth rates in nature. Bacteriol Rev. 1971 Mar;35(1):39–58. doi: 10.1128/br.35.1.39-58.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., Conrad H. E. Chondroitin SO4 catabolism in chick embryo chondrocytes. J Biol Chem. 1979 Apr 10;254(7):2316–2325. [PubMed] [Google Scholar]
- Macy J. M., Ljungdahl L. G., Gottschalk G. Pathway of succinate and propionate formation in Bacteroides fragilis. J Bacteriol. 1978 Apr;134(1):84–91. doi: 10.1128/jb.134.1.84-91.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Comparison of maintenance energy expenditures and growth yields among several rumen bacteria grown on continuous culture. Appl Environ Microbiol. 1979 Mar;37(3):537–543. doi: 10.1128/aem.37.3.537-543.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Arthur R., Kuritza A. Digestion of larch arabinogalactan by a strain of human colonic Bacteroides growing in continuous culture. J Agric Food Chem. 1981 May-Jun;29(3):475–480. doi: 10.1021/jf00105a009. [DOI] [PubMed] [Google Scholar]
- Salyers A. A., Kotarski S. F. Induction of chondroitin sulfate lyase activity in Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):781–788. doi: 10.1128/jb.143.2.781-788.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., O'Brien M. Cellular location of enzymes involved in chondroitin sulfate breakdown by Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):772–780. doi: 10.1128/jb.143.2.772-780.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. A reevaluation of the method for the determination of ATP production by measuring molar growth yields. Biochim Biophys Acta. 1973 Feb 12;301(1):53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]


