Abstract
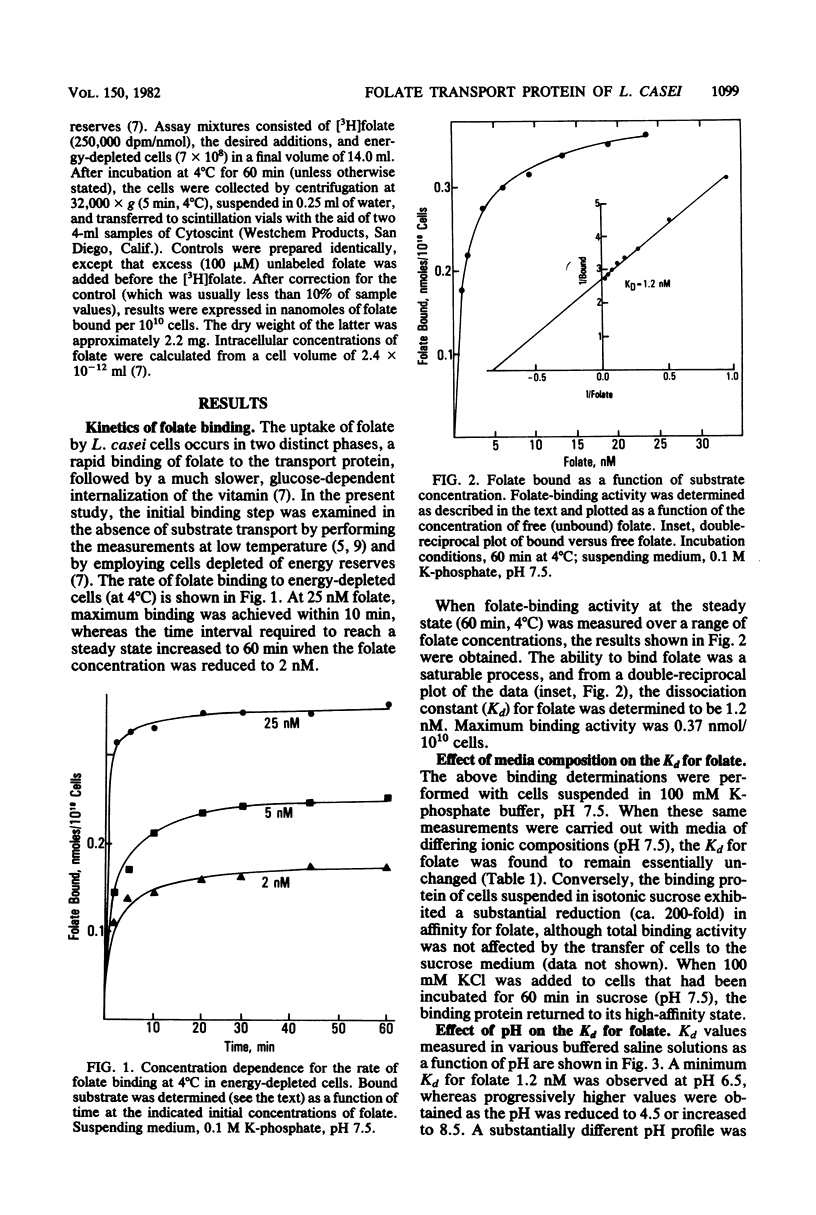
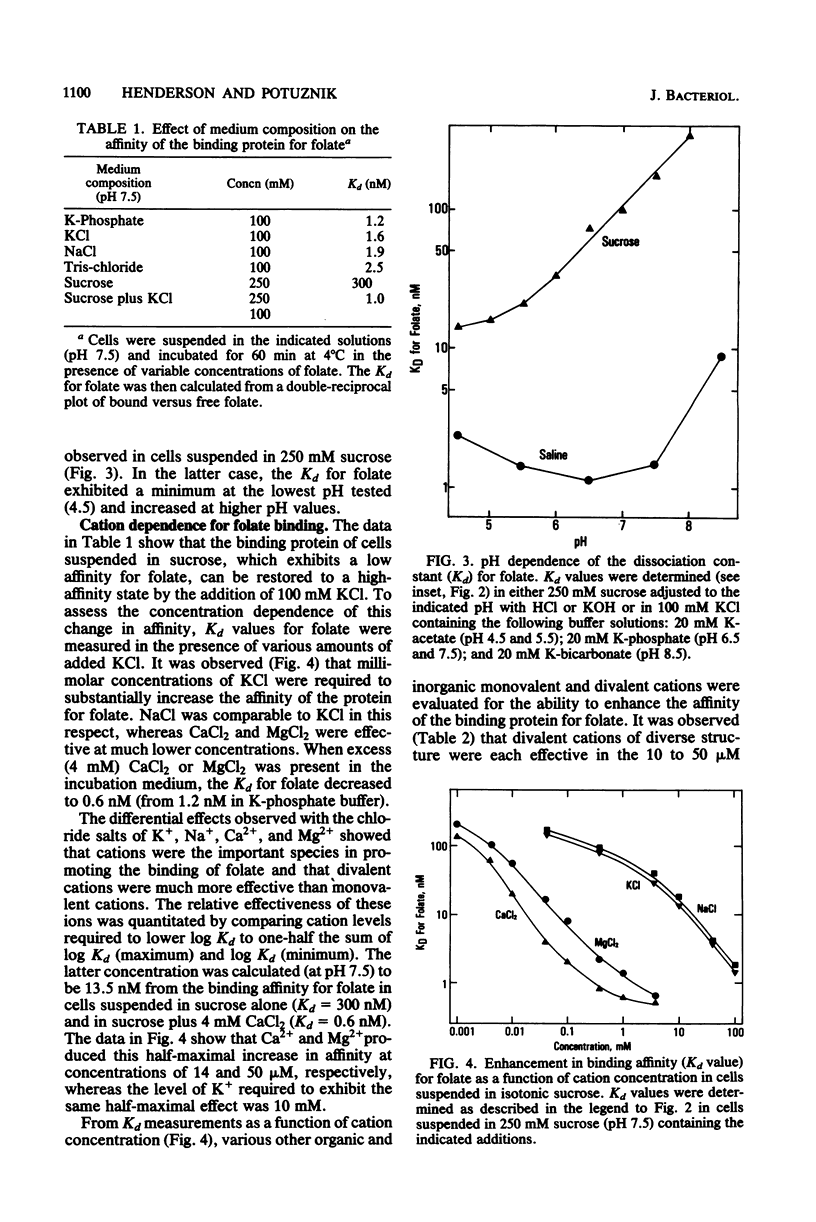
Lactobacillus casei cells grown in the presence of limiting folate contained large amounts of a membrane-associated binding protein which mediates folate transport. Binding to this protein at 4°C was time and concentration dependent and at low levels (1 to 10 nM) of folate required 60 min to reach a steady state. The apparent dissociation constant (Kd) for folate was 1.2 nM at pH 7.5 in 100 mM K-phosphate buffer, and it varied by less than twofold when measured over a range of pH values (5.5 to 7.5) or in buffered salt solutions of differing ionic compositions. Conversely, removal of ions and their replacement with isotonic sucrose (pH 7.5) led to a 200-fold reduction in binding affinity for folate. Restoration of the high-affinity state of the binding protein could be achieved by the readdition of various cations to the sucrose medium. Kd measurements over a range of cation concentrations revealed that a half-maximal restoration of binding affinity was obtained with relatively low levels (10 to 50 μM) of divalent cations (e.g., Ca2+, Mg2+, and ethylenediammonium2+ ions). Monovalent cations (e.g., Na+, K+, and Tris+) were also effective, but only at concentrations in the millimolar range. The Kd for folate reached a minimum of 0.6 nM at pH 7.5 in the presence of excess CaCl2. In cells suspended in sucrose, the affinity of the binding protein for folate increased 20-fold by decreasing the pH from 7.5 to 4.5, indicating that protons can partially fulfill the cation requirement. These results suggest that the folate transport protein of L. casei may contain both a substrate- and cation-binding site and that folate binds with a high affinity only after the cation-binding site has been occupied. The presence of these binding sites would support the hypothesis that folate is transported across the cell membrane via a cation-folate symport mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Friedberg I., Kaback H. R. Electrochemical proton gradient in Micrococcus lysodeikticus cells and membrane vesicles. J Bacteriol. 1980 May;142(2):651–658. doi: 10.1128/jb.142.2.651-658.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. I. The membrane potential. J Membr Biol. 1972;8(1):27–44. doi: 10.1007/BF01868093. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M. Anion exchange mechanism for transport of methotrexate in L1210 cells. Biochem Biophys Res Commun. 1981 Mar 16;99(1):163–169. doi: 10.1016/0006-291x(81)91727-7. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Huennekens F. M. Folate transport in Lactobacillus casei: solubilization and general properties of the binding protein. Biochem Biophys Res Commun. 1976 Feb 9;68(3):712–717. doi: 10.1016/0006-291x(76)91203-1. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Huennekens F. M. Mechanism of folate transport in Lactobacillus casei: evidence for a component shared with the thiamine and biotin transport systems. J Bacteriol. 1979 Mar;137(3):1308–1314. doi: 10.1128/jb.137.3.1308-1314.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Huennekens F. M. Purification and properties of a membrane-associated, folate-binding protein from Lactobacillus casei. J Biol Chem. 1977 Jun 10;252(11):3760–3765. [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Kadner R. J., Huennekens F. M. The folate and thiamine transport proteins of Lactobacillus casei. J Supramol Struct. 1977;6(2):239–247. doi: 10.1002/jss.400060209. [DOI] [PubMed] [Google Scholar]
- Huennekens F. M., Vitols K. S., Henderson G. B. Transport of folate compounds in bacterial and mammalian cells. Adv Enzymol Relat Areas Mol Biol. 1978;47:313–346. doi: 10.1002/9780470122921.ch5. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Shane B., Stokstad E. L. Transport and metabolism of folates by bacteria. J Biol Chem. 1975 Mar 25;250(6):2243–2253. [PubMed] [Google Scholar]
- West I. C. Energy coupling in secondary active transport. Biochim Biophys Acta. 1980 May 27;604(1):91–126. doi: 10.1016/0005-2736(80)90586-6. [DOI] [PubMed] [Google Scholar]
- Wilson D. B. Cellular transport mechanisms. Annu Rev Biochem. 1978;47:933–965. doi: 10.1146/annurev.bi.47.070178.004441. [DOI] [PubMed] [Google Scholar]


