Abstract
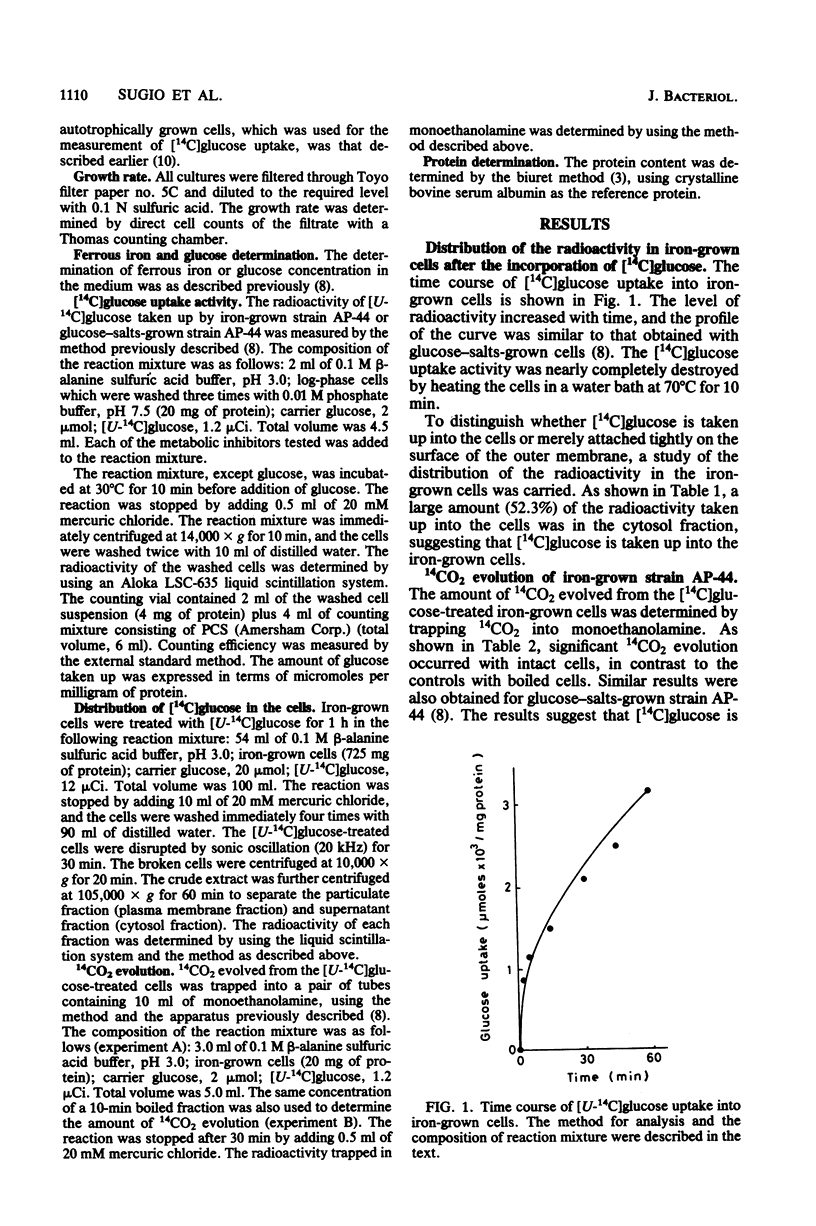
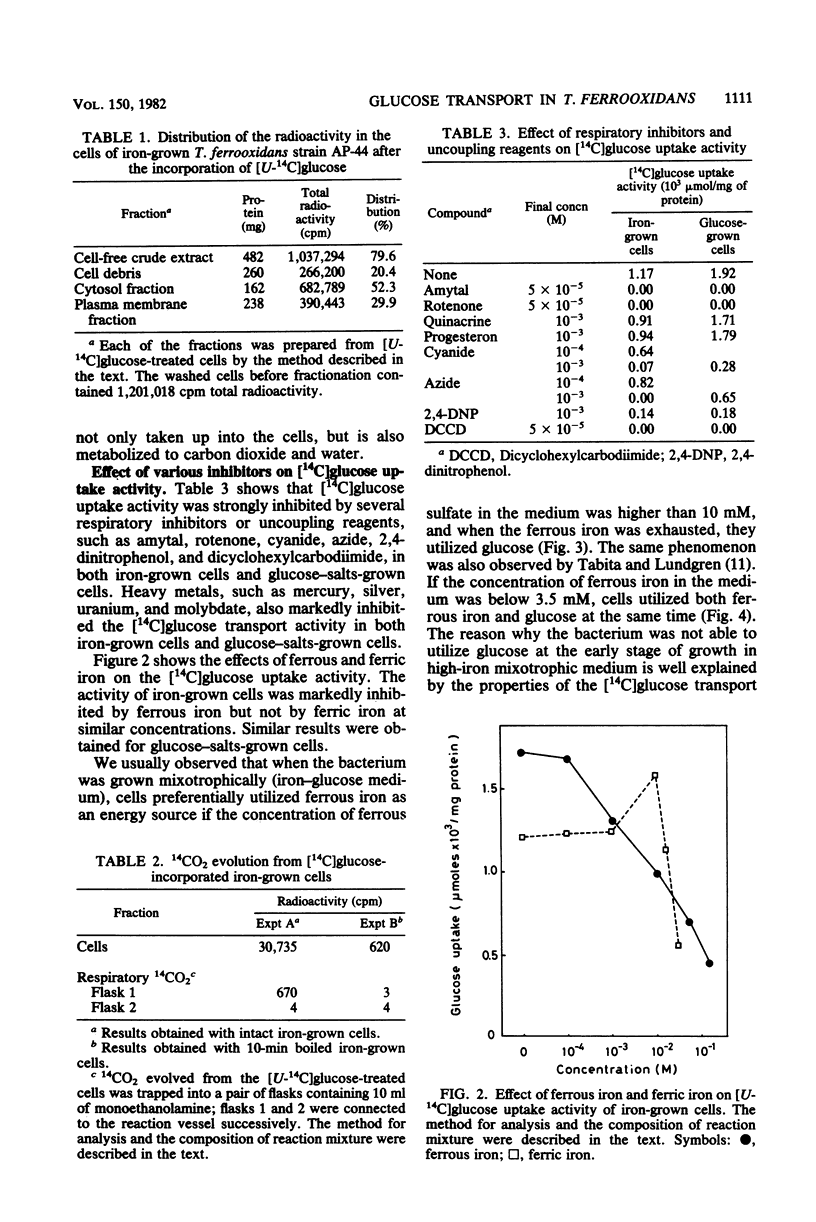
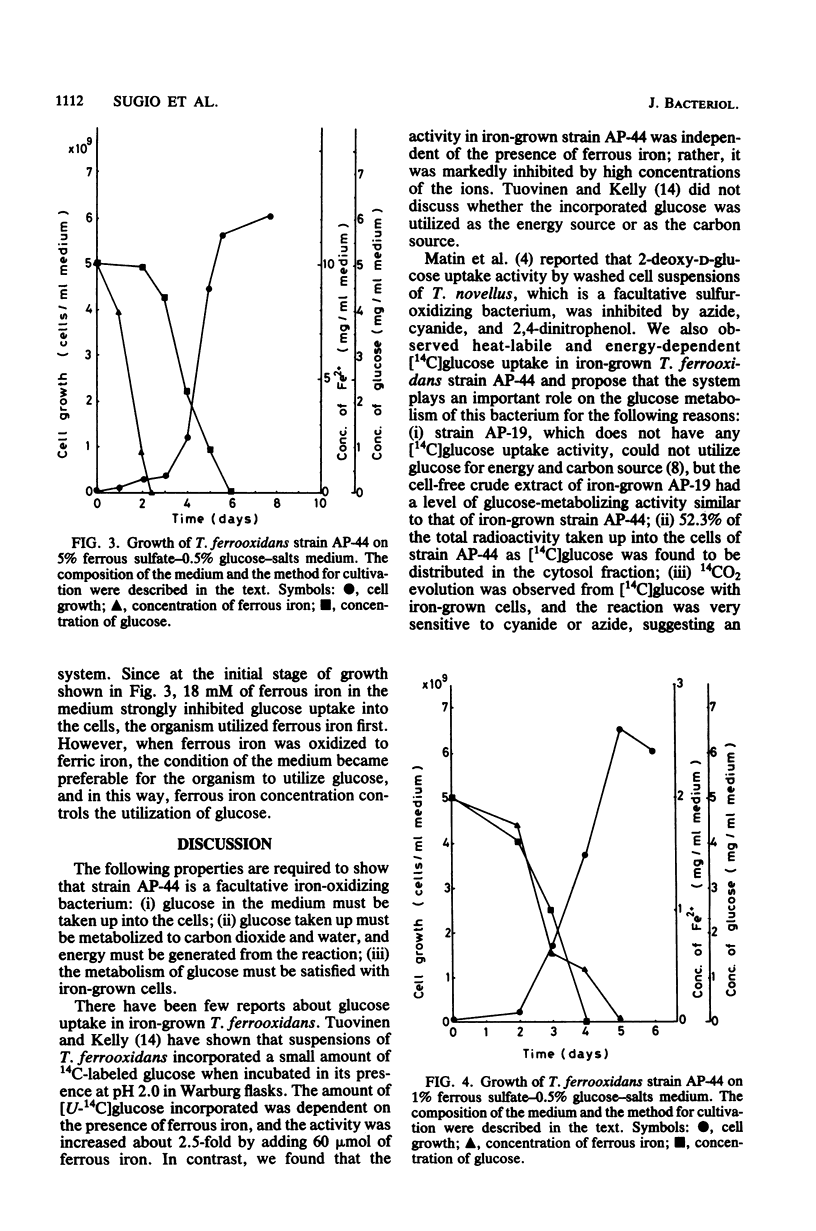
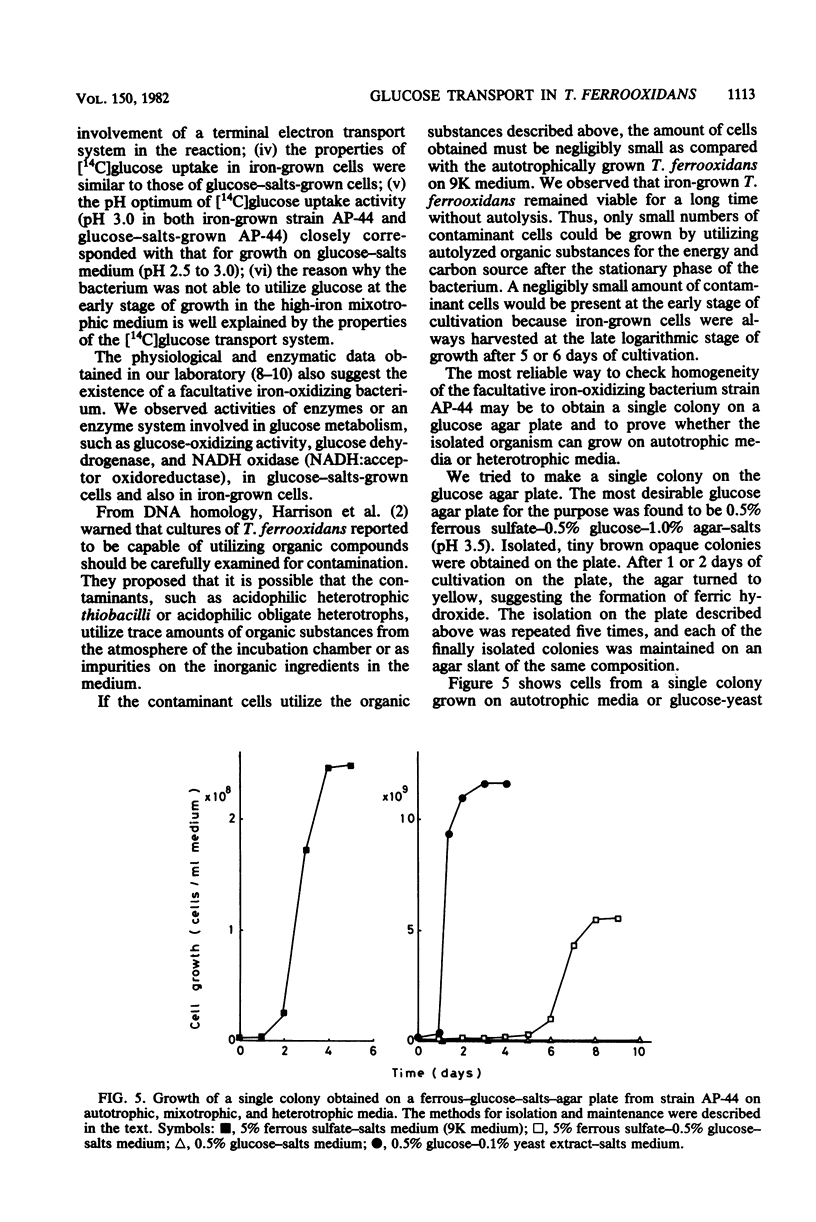
Properties of a heat-labile glucose transport system in Thiobacillus ferrooxidans strain AP-44 were investigated with iron-grown cells. [14C]glucose was incorporated into cell fractions, and the cells metabolized [14C]glucose to 14CO2. Amytal, rotenone, cyanide, azide, 2,4-dinitrophenol, and dicyclohexylcarbodiimide strongly inhibited [14C]glucose uptake activity, suggesting the presence of an energy-dependent glucose transport system in T. ferrooxidans. Heavy metals, such as mercury, silver, uranium, and molybdate, markedly inhibited the transport activity at 1 mM. When grown on mixotrophic medium, the bacteria preferentially utilized ferrous iron as an energy source. When iron was exhausted, the cells used glucose if the concentration of ferrous sulfate in the medium was higher than 3% (wt/vol). However, when ferrous sulfate was lower than 1%, both of the energy sources were consumed simultaneously.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Guay R., Silver M. Thiobacillus acidophilus sp. nov.; isolation and some physiological characteristics. Can J Microbiol. 1975 Mar;21(3):281–288. doi: 10.1139/m75-040. [DOI] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafia F., Brinson K. R., Heinzman M. W., Brady J. M. Transition of chemolithotroph Ferrobacillus ferrooxidans to obligate organotrophy and metabolic capabilities of glucose-grown cells. J Bacteriol. 1972 Jul;111(1):56–65. doi: 10.1128/jb.111.1.56-65.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita R., Lundgren D. G. Glucose-6-phosphate dehydrogenase from the chemolithotroph Thiobacillus ferrooxidans. J Bacteriol. 1971 Oct;108(1):343–352. doi: 10.1128/jb.108.1.343-352.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita R., Lundgren D. G. Utilization of glucose and the effect of organic compounds on the chemolithotroph Thiobacillus ferrooxidans. J Bacteriol. 1971 Oct;108(1):328–333. doi: 10.1128/jb.108.1.328-333.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuovinen O. H., Kelly D. P. Studies on the gorwth of Thiobacillus ferrooxidans. 3. Influence of uranium, other metal ions and 2:4-dinitrophenol on ferrous iron oxidation and carbon dioxide fixation by cell suspensions. Arch Mikrobiol. 1974 Feb 1;95(2):165–180. [PubMed] [Google Scholar]


