Abstract
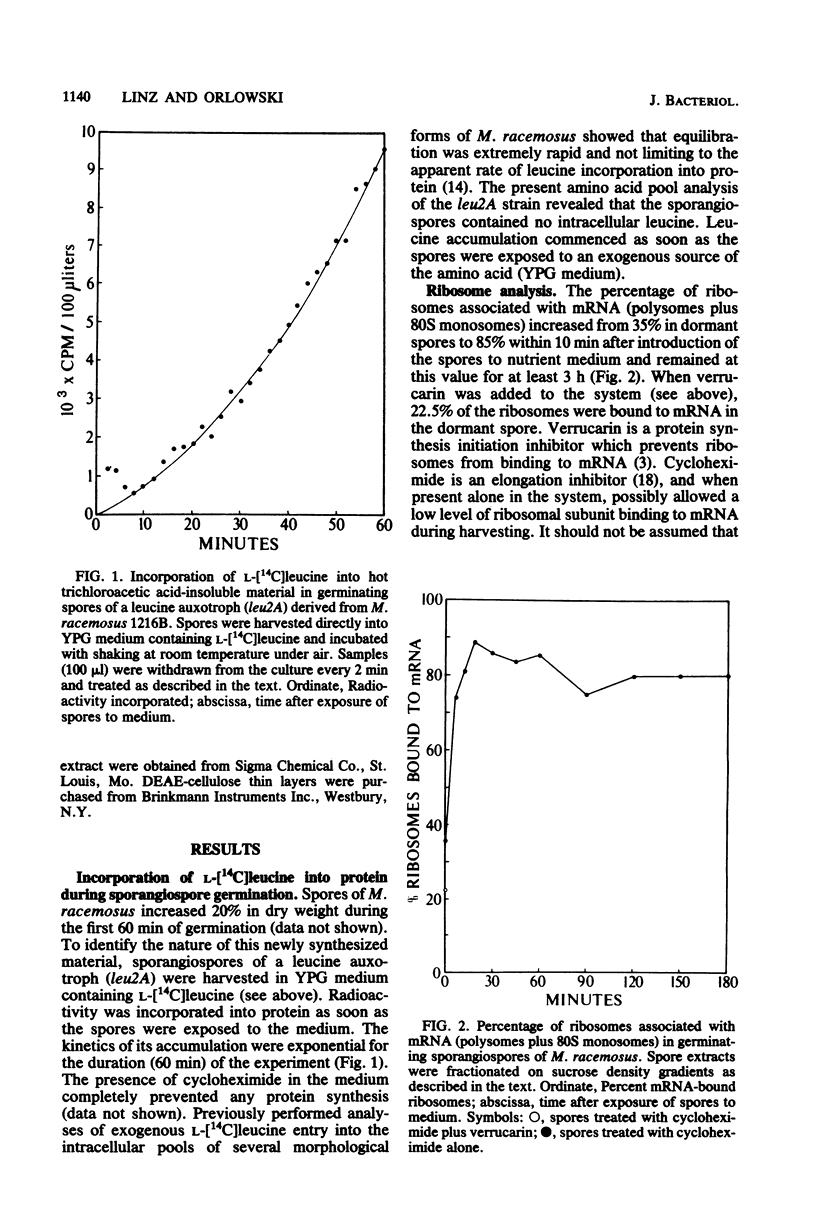
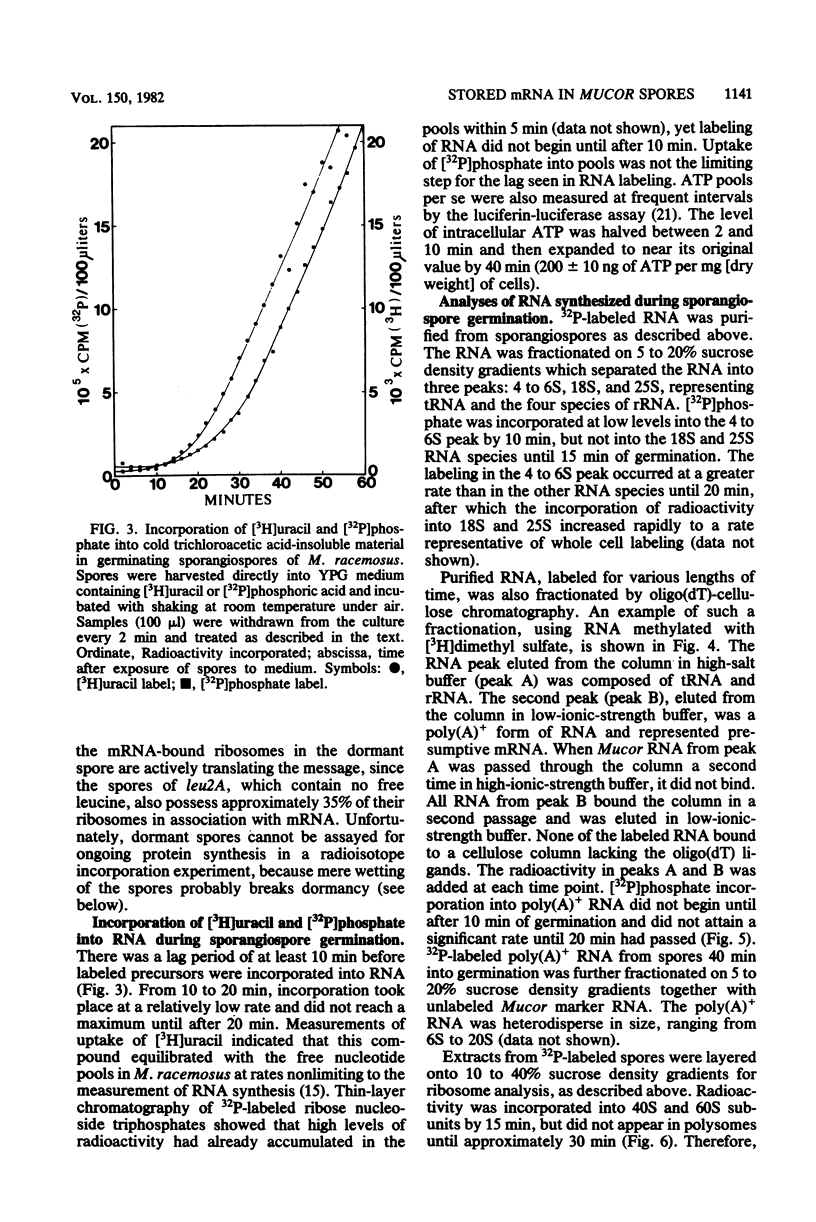
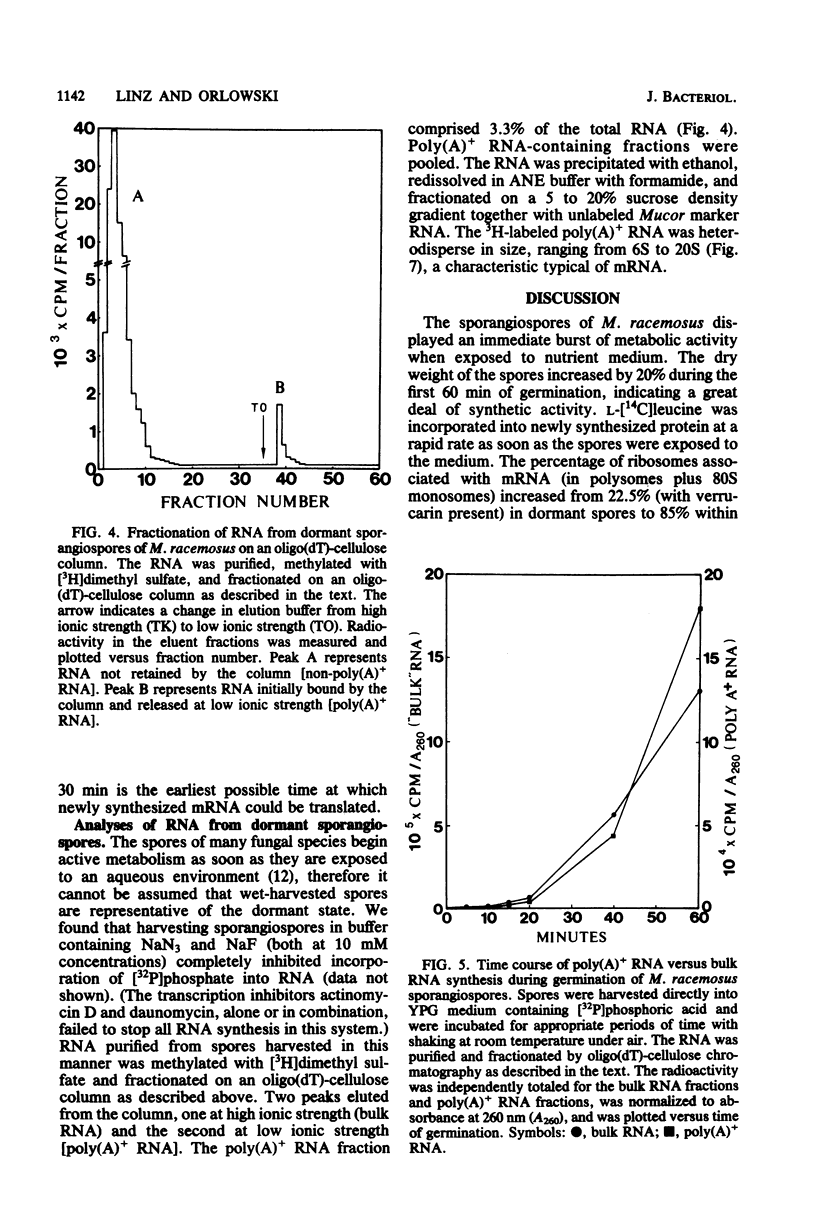
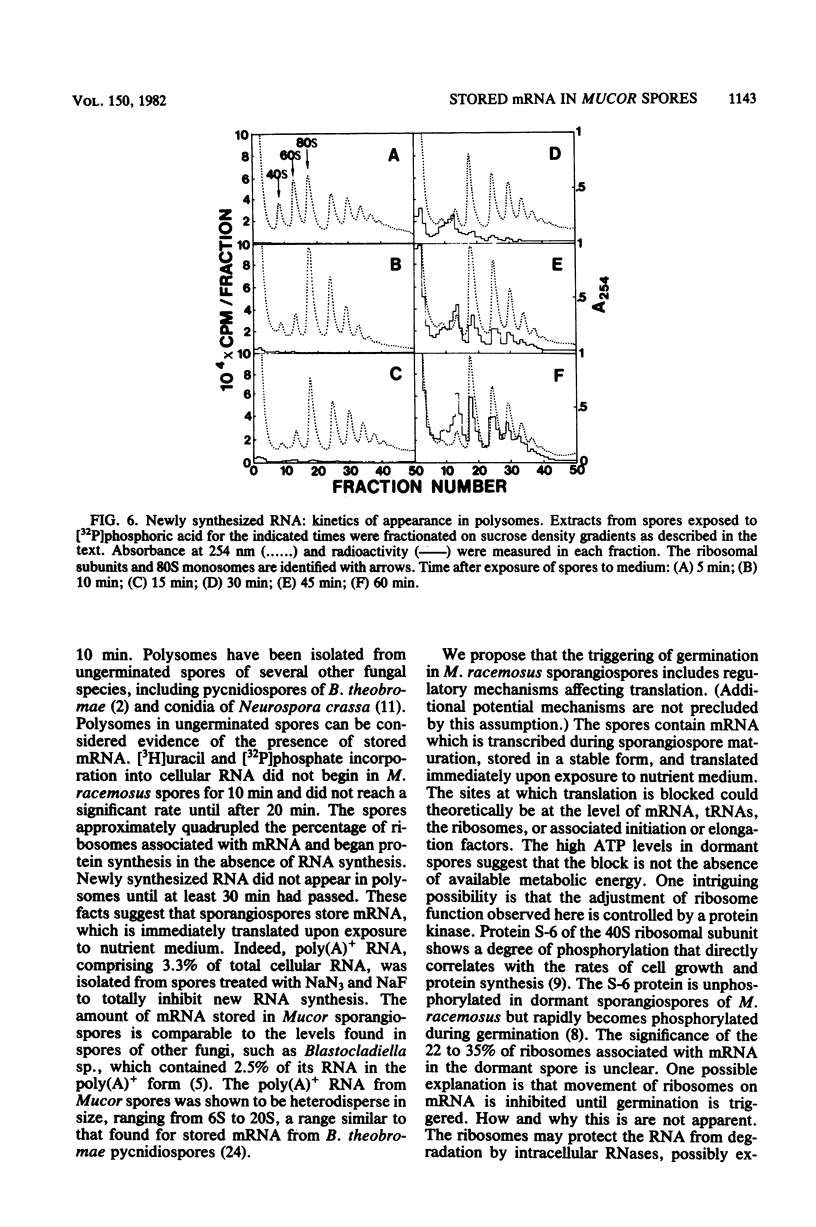
When introduced into nutrient medium under air, the asexual sporangiospores of Mucor racemosus germinated with 5 to 8 h, culminating with the emergence of germ tubes. We found that sporangiospores increased 20% in dry weight during the first 60 min of germination, indicating a high degree of synthetic activity. Sucrose density gradient analysis of spore extracts revealed that the percentage of ribosomes associated with mRNA increased from 22.5% in dormant spores to 85% within 10 min after the addition of medium and remained at this level for at least 3 h. L-[14C]leucine was immediately incorporated at a rapid rate into protein of a leucine auxotroph, whereas [3H]uracil or [32P]phosphate was incorporated into RNA at a significant rate only 20 min after the addition of medium. This newly synthesized RNA occurred in polysomes only after 30 min had passed. Pool synthesized RNA occurred in polysomes only after 30 min had passed. Pool equilibration of the radioactive precursors was not limiting to these measurements. Polyadenylated RNA was isolated from dormant spores by oligodeoxythymidylic acid-cellulose chromatography and was found to comprise 3.3% of the total cellular RNA. Sucrose density gradient centrifugation revealed the polyadenylated RNA to be heterodisperse in size, ranging from 6S to 20S. It was concluded that M. racemosus sporangiospores contain preformed mRNA which is translated commencing immediately upon the addition of nutrient medium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brambl R. Presence of polyribosomes in condiospores of Botryodiplodia theobromae harvested with nonaqueous solvents. J Bacteriol. 1975 Jun;122(3):1394–1395. doi: 10.1128/jb.122.3.1394-1395.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E., Cannon M., Davies J. Mechanism of inhibition of eukaryotic protein synthesis by trichothecene fungal toxins. Proc Natl Acad Sci U S A. 1974 Jan;71(1):30–34. doi: 10.1073/pnas.71.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. A., Lovett J. S., Wilt F. H. The polyadenylated RNA of zoospores and growth phase cells of the aquatic fungus, Blastocladiella. Dev Biol. 1977 Apr;56(2):329–342. doi: 10.1016/0012-1606(77)90274-3. [DOI] [PubMed] [Google Scholar]
- Knight R. H., Van Etten J. L. Characteristics of ribonucleic acids isolated for Botryodiplodia theobromae pycnidiospores. Arch Microbiol. 1976 Aug;109(1-2):45–50. doi: 10.1007/BF00425111. [DOI] [PubMed] [Google Scholar]
- Knight R. H., Van Etten J. L. Synthesis of ribonucleic acids during the germination of Botryodiplodia theobromae pycnidiospores. J Gen Microbiol. 1976 Aug;96(2):257–267. doi: 10.1099/00221287-95-2-257. [DOI] [PubMed] [Google Scholar]
- Larsen A., Sypherd P. Ribosomal proteins of the dimorphic fungus, Mucor racemosus. Mol Gen Genet. 1979 Aug;175(1):99–109. doi: 10.1007/BF00267861. [DOI] [PubMed] [Google Scholar]
- Orlowski M. Growth-rate-dependent adjustment of ribosome function in the fungus Mucor racemosus. Biochem J. 1981 May 15;196(2):403–410. doi: 10.1042/bj1960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Sypherd P. S. Protein synthesis during morphogenesis of Mucor racemosus. J Bacteriol. 1977 Oct;132(1):209–218. doi: 10.1128/jb.132.1.209-218.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Sypherd P. S. RNA synthesis during morphogenesis of the fungus Mucor racemosus. Arch Microbiol. 1978 Nov 13;119(2):145–152. doi: 10.1007/BF00964265. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Sypherd P. S. Regulation of macromolecular synthesis during hyphal germ tube emergence from Mucor racemosus sporangiospores. J Bacteriol. 1978 Apr;134(1):76–83. doi: 10.1128/jb.134.1.76-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Sypherd P. S. Regulation of translation rate during morphogenesis in the fungus Mucor. Biochemistry. 1978 Feb 21;17(4):569–575. doi: 10.1021/bi00597a002. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Smith B. A., Burke D. D. Evidence for the presence of messenger ribonucleic acid in Allomyces macrogynus mitospores. J Bacteriol. 1979 May;138(2):535–541. doi: 10.1128/jb.138.2.535-541.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Sypherd P. S., Borgia P. T., Paznokas J. L. Biochemistry of dimorphism in the fungus Mucor. Adv Microb Physiol. 1978;18:67–104. doi: 10.1016/s0065-2911(08)60415-4. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Rawn C. D. Polyadenylate-containing RNA in dormant and germinating Botryodiplodia theobromae pycnidiospores. Can J Microbiol. 1979 Mar;25(3):375–379. doi: 10.1139/m79-058. [DOI] [PubMed] [Google Scholar]


