Abstract
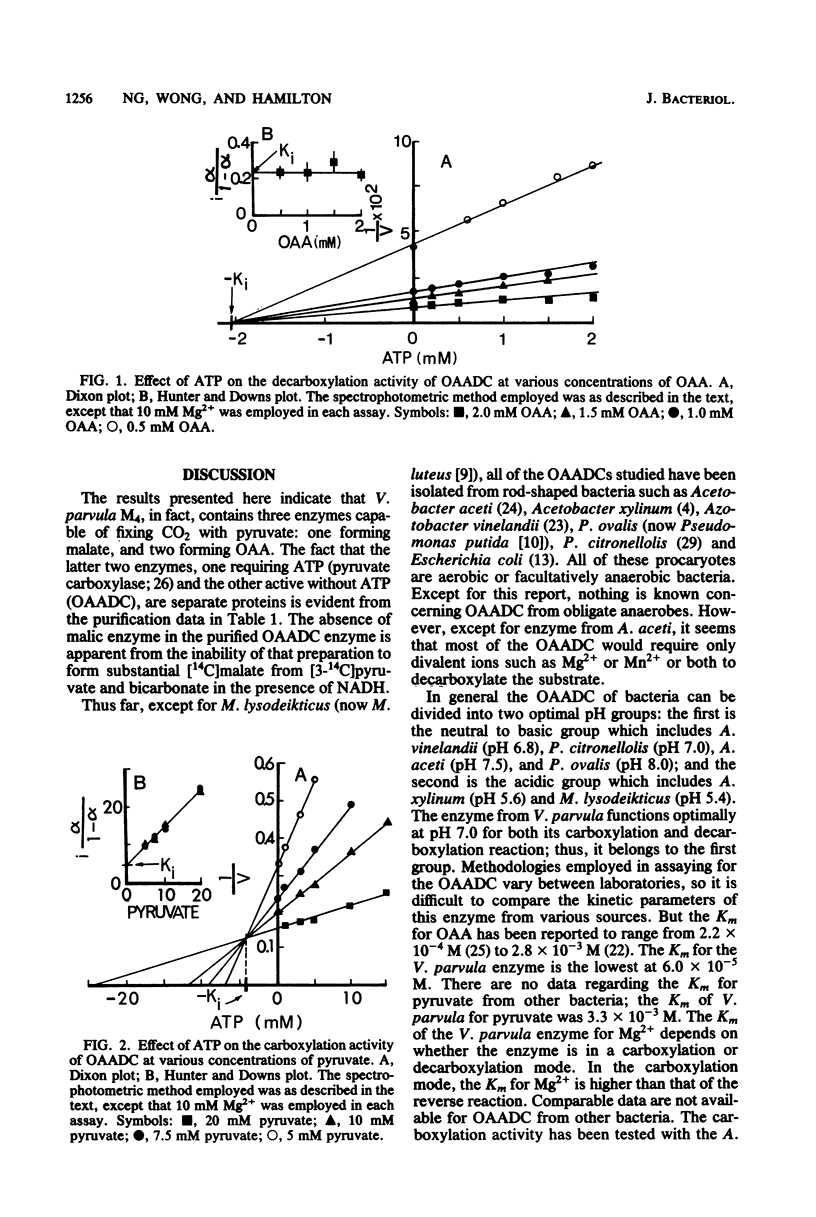
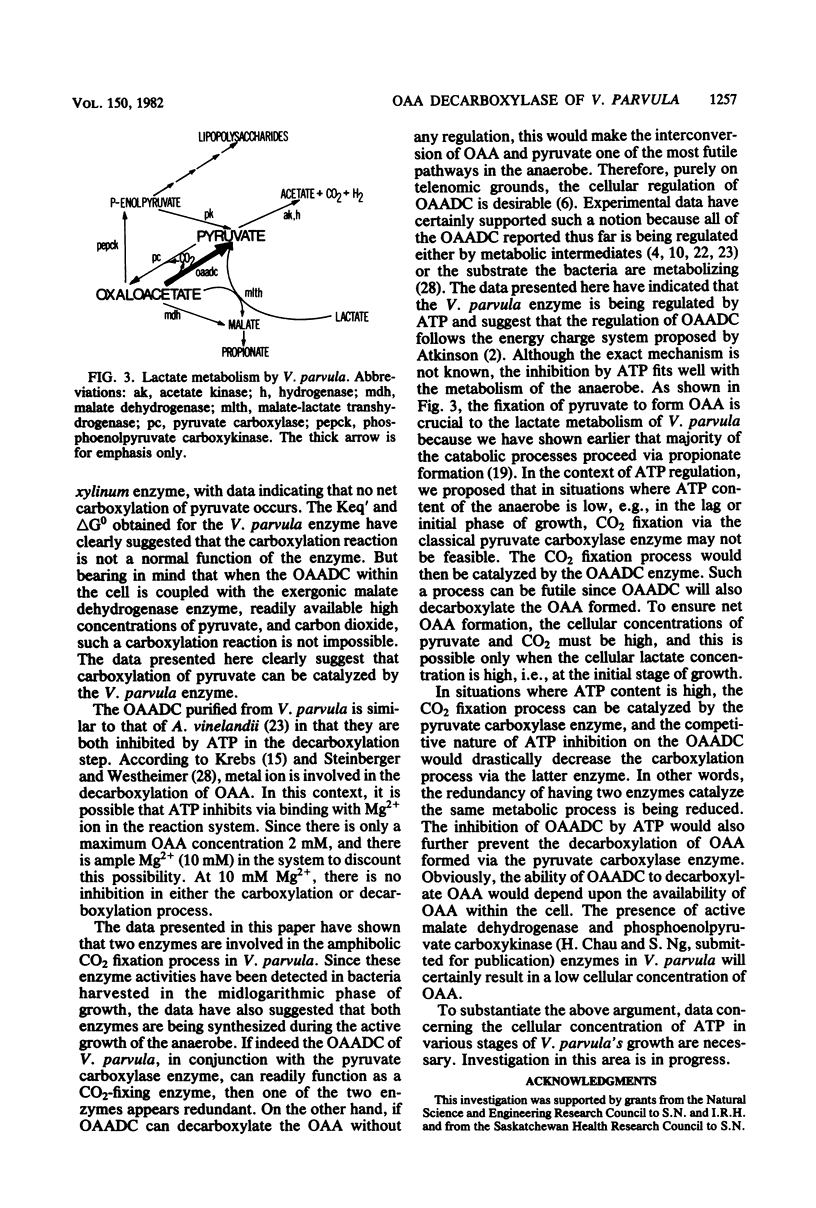
Oxaloacetate decarboxylase was purified to 136-fold from the oral anaerobe Veillonella parvula. The purified enzyme was substantially free of contaminating enzymes or proteins. Maximum activity of the enzyme was exhibited at pH 7.0 for both carboxylation and decarboxylation. At this pH, the Km values for oxaloacetate and Mg2+ were at 0.06 and 0.17 mM, respectively, whereas the Km values for pyruvate, CO2, and Mg2+ were 3.3, 1.74, and 1.85 mM, respectively. Hyperbolic kinetics were observed with all of the aforementioned compounds. The Keq' was 2.13 X 10(-3) mM-1 favoring the decarboxylation of oxaloacetate. In the carboxylation step, avidin, acetyl coenzyme A, biotin, and coenzyme A were not required. ADP and NADH had no effect on either the carboxylation or decarboxylation step, but ATP inhibited the carboxylation step competitively and the decarboxylation step noncompetitively. These types of inhibition fitted well with the overall lactate metabolism of the non-carbohydrate-fermenting anaerobe.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. H. The isolation and characterization of malate-lactate transhydrogenase from Micrococcus lactilyticus. J Biol Chem. 1966 Nov 25;241(22):5266–5275. [PubMed] [Google Scholar]
- BENZIMAN M., HELLER N. OXALOACETATE DECARBOXYLATION AND OXALOACETATE-CARBON DIOXIDE EXCHANGE IN ACETOBACTER XYLINUM. J Bacteriol. 1964 Dec;88:1678–1687. doi: 10.1128/jb.88.6.1678-1687.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D. The teleonomic significance of biosynthetic control mechanisms. Cold Spring Harb Symp Quant Biol. 1961;26:1–10. doi: 10.1101/sqb.1961.026.01.005. [DOI] [PubMed] [Google Scholar]
- HORTON A. A., KORNBERG H. L. OXALOACETATE 4-CARBOXY-LYASE FROM PSEUDOMONAS OVALIS CHESTER. Biochim Biophys Acta. 1964 Aug 26;89:381–383. doi: 10.1016/0926-6569(64)90236-6. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. The effect of inorganic salts on the ketone decomposition of oxaloacetic acid. Biochem J. 1942 Apr;36(3-4):303–305. doi: 10.1042/bj0360303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ng S. K., Hamilton I. R. Carbon dioxide fixation by Veillonella parvula M 4 and its relation to propionic acid formation. Can J Microbiol. 1973 Jun;19(6):715–723. doi: 10.1139/m73-116. [DOI] [PubMed] [Google Scholar]
- Ng S. K., Hamilton I. R. Gluconeogenesis by Veillonella parvula M4: evidence for the indirect conversion of pyruvate to P-enolpyruvate. Can J Microbiol. 1974 Jan;20(1):19–28. doi: 10.1139/m74-004. [DOI] [PubMed] [Google Scholar]
- Ng S. K., Hamilton I. R. Lactate metabolism by Veillonella parvula. J Bacteriol. 1971 Mar;105(3):999–1005. doi: 10.1128/jb.105.3.999-1005.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R., Chuang D. T., Taylor B. L., Utter M. F. Novel enzymic machinery for the metabolism of oxalacetate, phosphoenolpyruvate, and pyruvate in Pseudomonas citronellolis. J Biol Chem. 1977 Feb 25;252(4):1257–1263. [PubMed] [Google Scholar]
- ROGOSA M. A selective medium for the isolation and enumeration of the veillonella from the oral cavity. J Bacteriol. 1956 Oct;72(4):533–536. doi: 10.1128/jb.72.4.533-536.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITELEY H. R., McCORMICK N. G. Degradation of pyruvate by Micrococcus lactilyticus. III. Properties and cofactor requirements of the carbon dioxide-exchange reaction. J Bacteriol. 1963 Feb;85:382–393. doi: 10.1128/jb.85.2.382-393.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


