Abstract
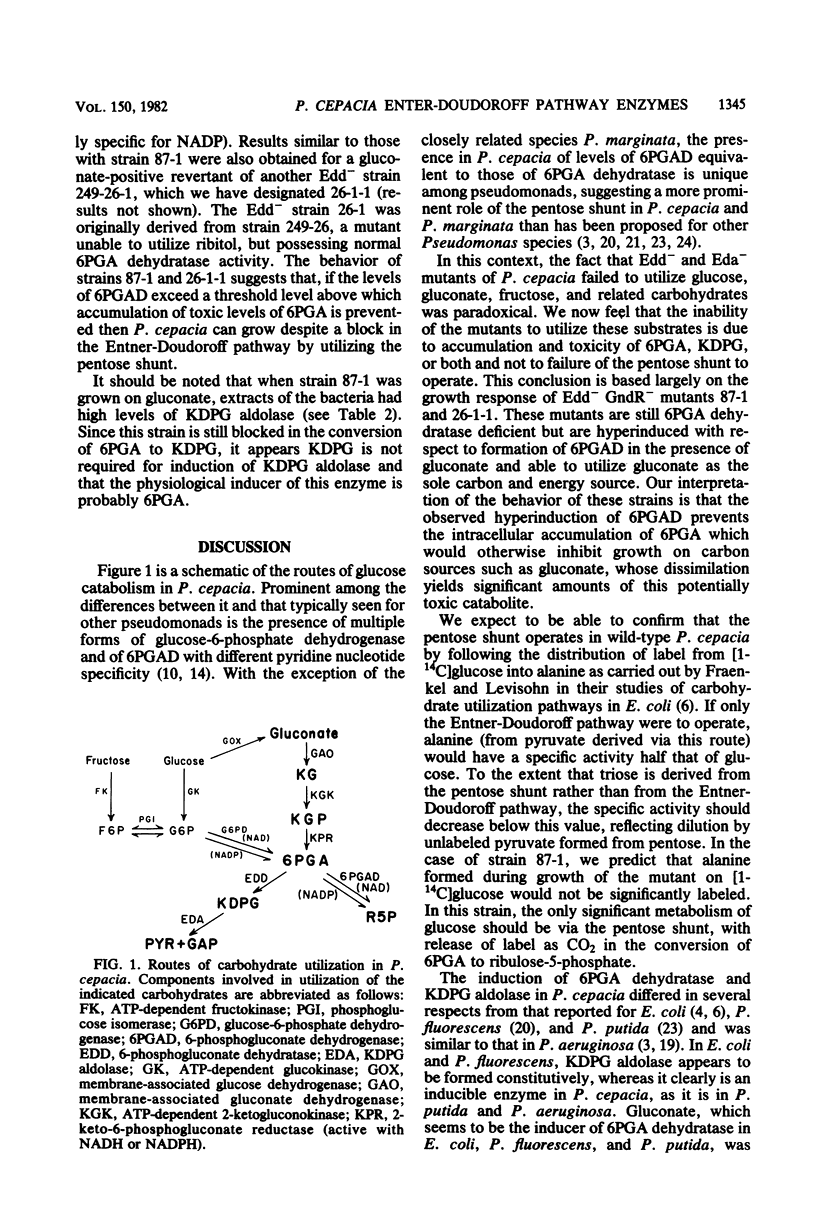
Pseudomonas cepacia mutants deficient in either 6-phosphogluconate (6PGA) dehydratase (Edd-) or 2-keto-3-deoxy-6-phosphogluconate (KDPG) aldolase (Eda-) failed to utilize glucose or gluconate despite the prominence of of 6-phosphogluconate dehydrogenase (6PGAD) ii this bacterium and the potential for utilizing the pentose shunt suggested by its growth on ribitol and xylose. The Eda- strains grew normally on glucuronic acid, indicating that in P. cepacia its degradation does not depend upon KDPG aldolase as it does in Escherichia coli. Both 6PGA dehydratase and KDPG aldolase were inducible enzymes, with 6PGA rather than gluconate the apparent inducer. Edd- as well as Eda- strains were sensitive to growth inhibition by glucose, gluconate, fructose, and related carbohydrates when these substrates were present in combination with alternate carbon sources such as citrate or phthalate, presumably as a consequence of accumulation and toxicity of 6PGA, KDPG, or both. Edd- mutants were somewhat less sensitive to such inhibition than were Eda- strains. Certain derivatives of the Edd- strains we examined were able to utilize gluconate despite their deficiency of 6PGA dehydratase. Such mutants formed higher levels of 6PGAD than did the wild type. It is likely that the elevated levels of 6PGAD in these strains prevents accumulation of toxic levels of 6PGA that would otherwise result from a block in he Entner-Doudoroff pathway. The results suggest that P. cepacia can mutate to grow slowly on gluconate utilizing only the pentose shunt.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman W., Lessie T. G. Response of Pseudomonas cepacia to beta-Lactam antibiotics: utilization of penicillin G as the carbon source. J Bacteriol. 1979 Dec;140(3):1126–1128. doi: 10.1128/jb.140.3.1126-1128.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins W. T., Feary T. W., Phibbs P. V., Jr 6-Phosphogluconate dehydratase deficiency in pleiotropic carbohydrate-negative mutant strains of Pseudomonas aeruginosa. J Bacteriol. 1975 Mar;121(3):942–949. doi: 10.1128/jb.121.3.942-949.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. C., Dobrogosz W. J. Gluconate metabolism in Escherichia coli. J Bacteriol. 1967 Mar;93(3):941–949. doi: 10.1128/jb.93.3.941-949.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradkin J. E., Fraenkel D. G. 2-keto-3-deoxygluconate 6-phosphate aldolase mutants of Escherichia coli. J Bacteriol. 1971 Dec;108(3):1277–1283. doi: 10.1128/jb.108.3.1277-1283.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G., Levisohn S. R. Glucose and gluconate metabolism in an Escherichia coli mutant lacking phosphoglucose isomerase. J Bacteriol. 1967 May;93(5):1571–1578. doi: 10.1128/jb.93.5.1571-1578.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath H. E., Gaudy E. T. Relationship between catabolism of glycerol and metabolism of hexosephosphate derivatives by Pseudomonas aeruginosa. J Bacteriol. 1978 Nov;136(2):638–646. doi: 10.1128/jb.136.2.638-646.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOVACHEVICH R., WOOD W. A. Carbohydrate metabolism by Pseudomonas fluorescens. III. Purification and properties of a 6-phosphogluconate dehydrase. J Biol Chem. 1955 Apr;213(2):745–756. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee Y. N., Lessie T. G. Purification and characterization of the two 6-phosphogluconate dehydrogenase species from Pseudomonas multivorans. J Bacteriol. 1974 Dec;120(3):1043–1057. doi: 10.1128/jb.120.3.1043-1057.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessie T. G., Berka T., Zamanigian S. Pseudomonas cepacia mutants blocked in the direct oxidative pathway of glucose degradation. J Bacteriol. 1979 Jul;139(1):323–325. doi: 10.1128/jb.139.1.323-325.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessie T. G., Whiteley H. R. Properties of threonine deaminase from a bacterium able to use threonine as sole source of carbon. J Bacteriol. 1969 Nov;100(2):878–889. doi: 10.1128/jb.100.2.878-889.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessie T. G., Wyk J. C. Multiple forms of Pseudomonas multivorans glucose-6-phosphate and 6-phosphogluconate dehydrogenases: differences in size, pyridine nucleotide specificity, and susceptibility to inhibition by adenosine 5'-triphosphate. J Bacteriol. 1972 Jun;110(3):1107–1117. doi: 10.1128/jb.110.3.1107-1117.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessie T., Neidhardt F. C. Adenosine triphosphate-linked control of Pseudomonas aeruginosa glucose-6-phosphate dehydrogenase. J Bacteriol. 1967 Apr;93(4):1337–1345. doi: 10.1128/jb.93.4.1337-1345.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Phibbs P. V., Jr, McCowen S. M., Feary T. W., Blevins W. T. Mannitol and fructose catabolic pathways of Pseudomonas aeruginosa carbohydrate-negative mutants and pleiotropic effects of certain enzyme deficiencies. J Bacteriol. 1978 Feb;133(2):717–728. doi: 10.1128/jb.133.2.717-728.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Friedman S. B., Eisenberg R. C. Gluconate regulation of glucose catabolism in Pseudomonas fluorescens. J Bacteriol. 1972 Oct;112(1):291–298. doi: 10.1128/jb.112.1.291-298.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer M. H., Baumann P., Baumann L., Berman S. M., Cánovas J. L., Berman R. H. Pathways of D-fructose catabolism in species of Pseudomonas. Arch Microbiol. 1977 Feb 4;112(1):49–55. doi: 10.1007/BF00446653. [DOI] [PubMed] [Google Scholar]
- Wong H. C., Lessie T. G. Hydroxy amino acid metabolism in Pseudomonas cepacia: role of L-serine deaminase in dissimilation of serine, glycine, and threonine. J Bacteriol. 1979 Oct;140(1):240–245. doi: 10.1128/jb.140.1.240-245.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


