Abstract
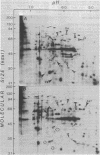
Polypeptides of whole-cell extracts of Naegleria fowleri flagellates and growing amebae were resolved by two-dimensional polyacrylamide gel electrophoresis. Autoradiograms of the [35S]methionine-labeled polypeptides of amebae and flagellates were analyzed by two dimensional densitometry to determine whether there were correlations between intracellular concentration of a protein and subunit size or charge. The majority of the polypeptides of amebae and flagellates had molecular sizes in the range of 20 to 60 kilodaltons. The radioactivity per polypeptide species in the size range of 20 to 60 kilodaltons was greater in amebae than in flagellates. The greatest number of polypeptides detected in amebae and flagellates was in the isoelectric focusing range of pH 6 to 7. The radioactivity per polypeptide species in the isoelectric focusing gradient below 6.3 was greater in amebae than in flagellates. Polypeptides in the size range of 20 to 60 kilodaltons had a median isoelectric point below pI 6.3, whereas those larger than 60 kilodaltons had a median pI value above 6.3. These data indicated that molecular size and charge were not entirely independent variables and that the size and charge of a polypeptide might have an important influence in determining its intracellular concentration in both amebae and flagellates. Autoradiograms were also compared so that changes in intracellular protein complement and concentrations occurring during differentiation could be recognized. The relative amounts of a limited number of polypeptides increased markedly, and others decreased markedly, during enflagellation.
Full text
PDF

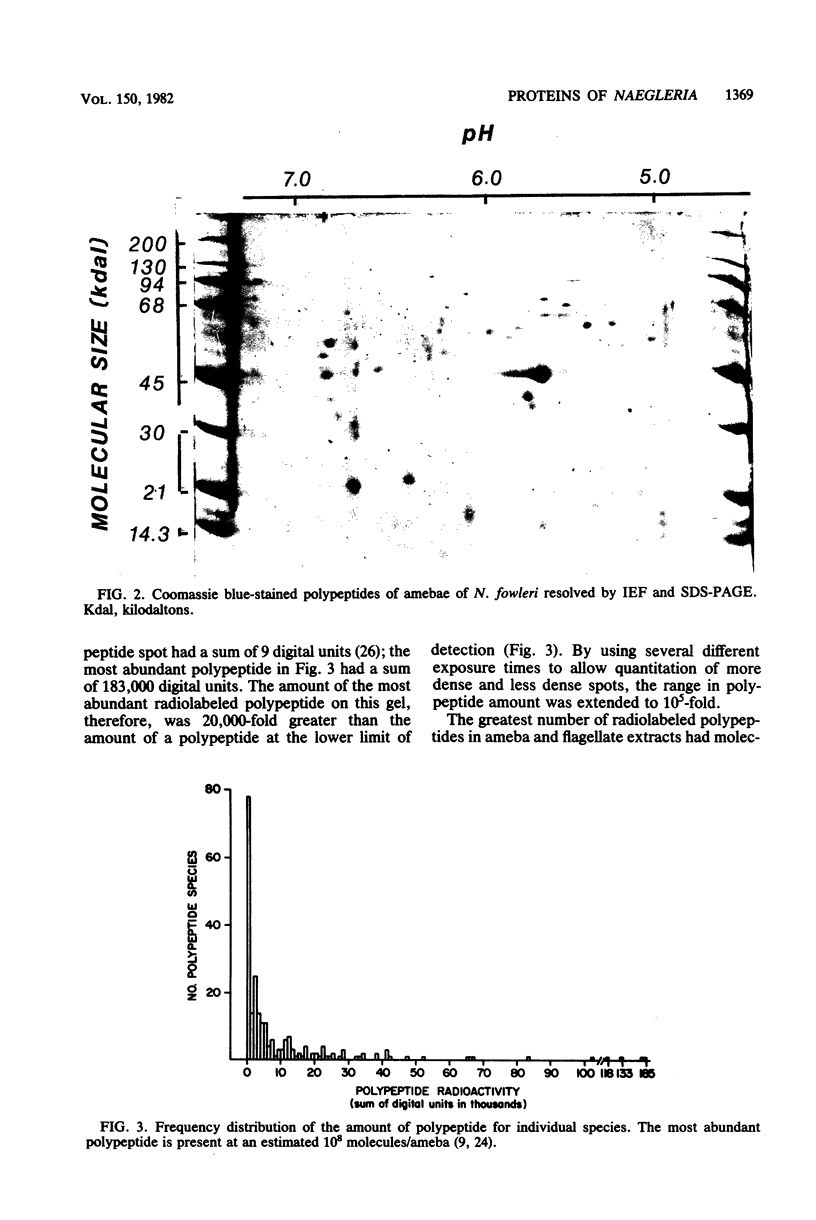
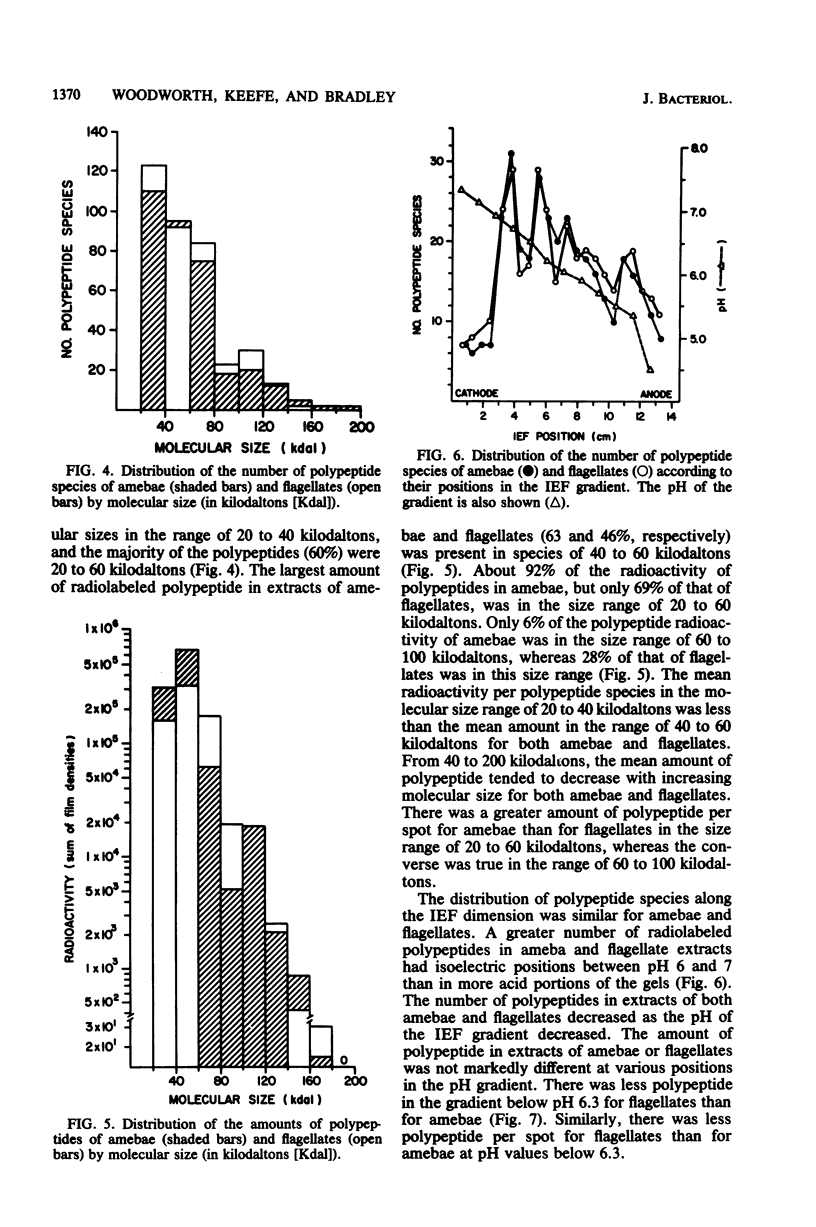
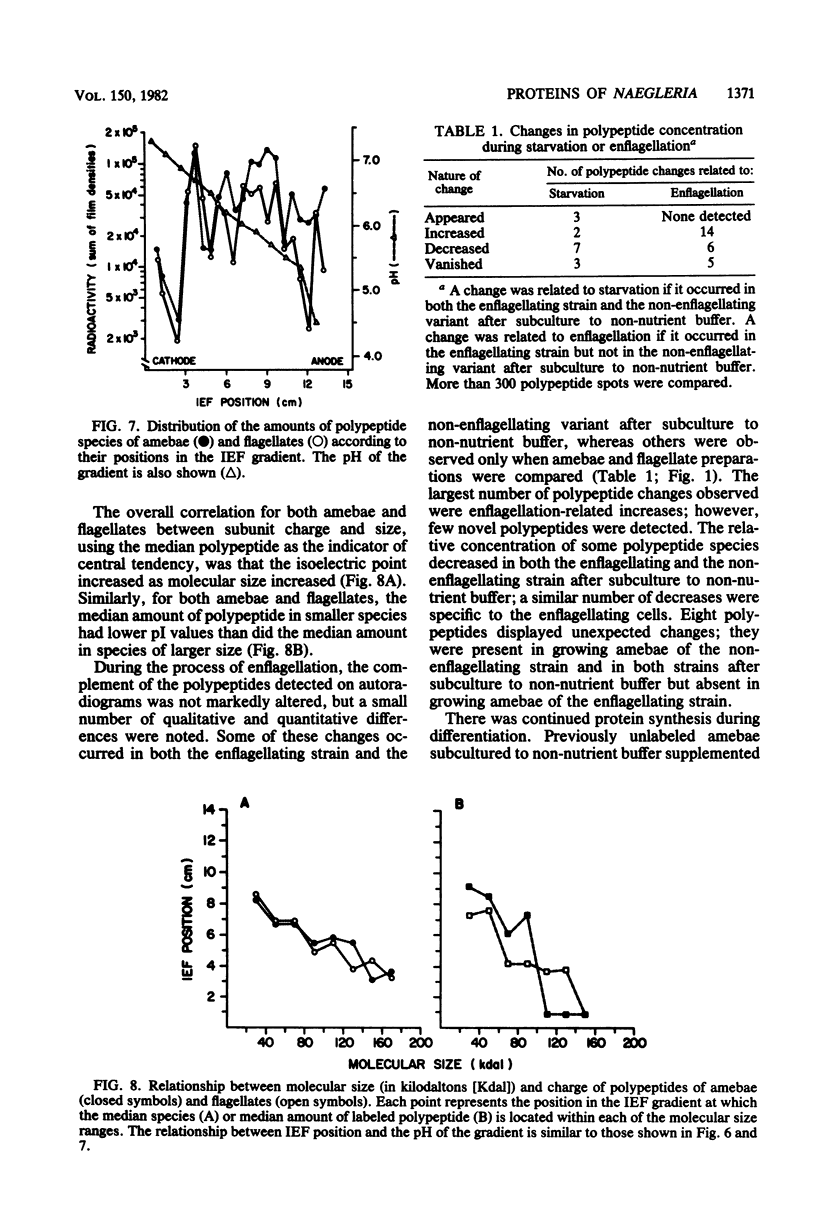
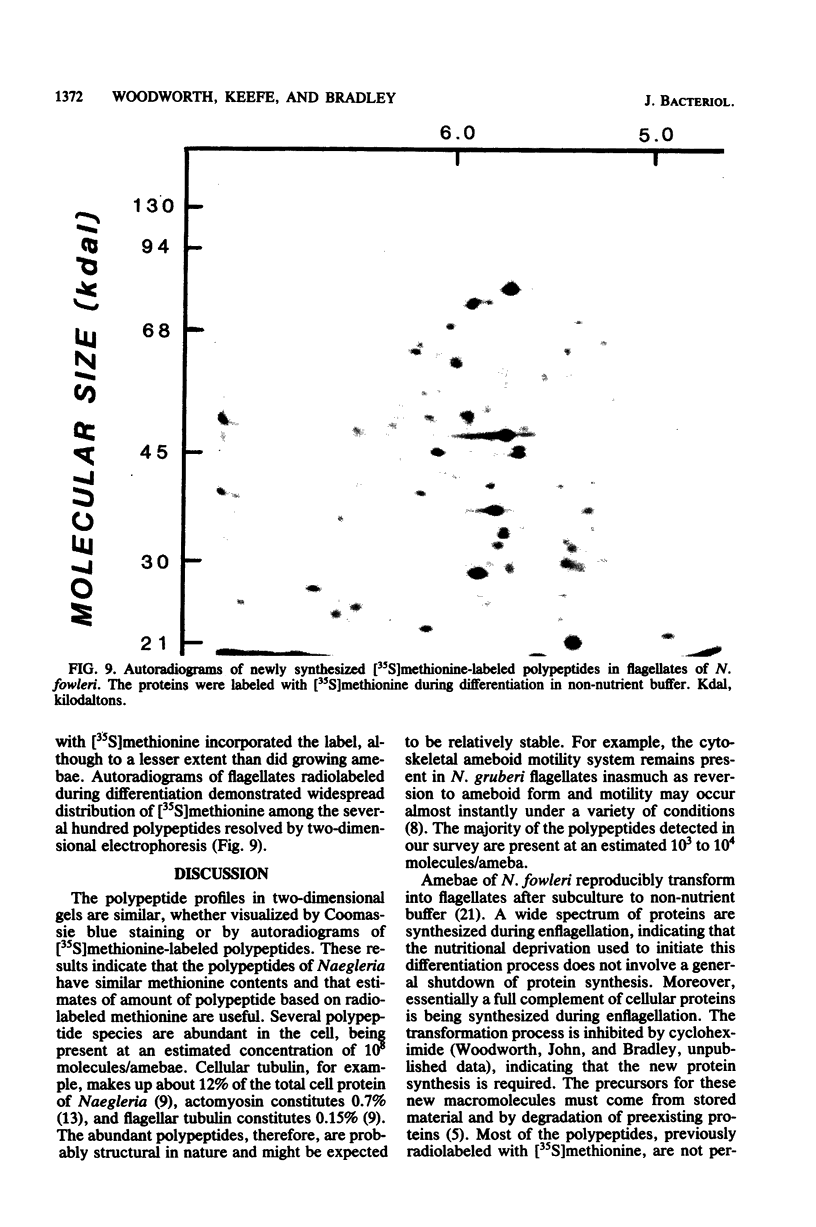


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley S. G., Bond J. S. Taxonomic criteria for Mycobacteria and nocardiae. Adv Appl Microbiol. 1974;18(0):131–190. doi: 10.1016/s0065-2164(08)70571-9. [DOI] [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. Relationship between in vivo degradative rates and isoelectric points of proteins. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3893–3897. doi: 10.1073/pnas.72.10.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice J. F., Hess E. J., Goldberg A. L. Studies on the relationship between the degradative rates of proteins in vivo and their isoelectric points. Biochem J. 1979 Feb 15;178(2):305–312. doi: 10.1042/bj1780305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus J. C., Kahn A., Schapira F. Posttranslational modifications of enzymes. Curr Top Cell Regul. 1978;14:243–297. doi: 10.1016/b978-0-12-152814-0.50010-1. [DOI] [PubMed] [Google Scholar]
- Duncan W. E., Offermann M. K., Bond J. S. Intracellular turnover of stable and labile soluble liver proteins. Arch Biochem Biophys. 1980 Feb;199(2):331–341. doi: 10.1016/0003-9861(80)90288-x. [DOI] [PubMed] [Google Scholar]
- Fulton C. Cell differentiation in Naegleria gruberi. Annu Rev Microbiol. 1977;31:597–629. doi: 10.1146/annurev.mi.31.100177.003121. [DOI] [PubMed] [Google Scholar]
- Fulton C., Kowit J. D. Programmed synthesis of flagellar tubulin during cell differentiation in Naegleria. Ann N Y Acad Sci. 1975 Jun 30;253:318–332. doi: 10.1111/j.1749-6632.1975.tb19210.x. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Haight J. B., John D. T. Growth of Naegleria fowleri in several axenic media. Folia Parasitol (Praha) 1980;27(3):207–212. [PubMed] [Google Scholar]
- Hiatt W. R., Inderlied C. B., Sypherd P. S. Differential synthesis of polypeptides during morphogenesis of Mucor. J Bacteriol. 1980 Mar;141(3):1350–1359. doi: 10.1128/jb.141.3.1350-1359.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H., O'Farrell P. Z. Two-dimensional polyacrylamide gel electrophoretic fractionation. Methods Cell Biol. 1977;16:407–420. doi: 10.1016/s0091-679x(08)60116-8. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pacheco G., Tulloch G. S. Microfilariae of Dirofilaria striata in a dog. J Parasitol. 1970 Apr;56(2):248–248. [PubMed] [Google Scholar]
- Page F. C. Taxonomic criteria for limax amoebae, with descriptions of 3 new species of Hartmannella and 3 of Vahlkampfia. J Protozool. 1967 Aug;14(3):499–521. doi: 10.1111/j.1550-7408.1967.tb02036.x. [DOI] [PubMed] [Google Scholar]
- Paskin N., Mayer R. J. The role of enzyme degradation in enzyme turnover during tissue differentiation. Biochim Biophys Acta. 1977 Jan 3;474(1):1–10. doi: 10.1016/0005-2787(77)90208-8. [DOI] [PubMed] [Google Scholar]
- Patterson M., Woodworth T. W., Marciano-Cabral F., Bradley S. G. Ultrastructure of Naegleria fowleri enflagellation. J Bacteriol. 1981 Jul;147(1):217–226. doi: 10.1128/jb.147.1.217-226.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trew B. J., Friesen J. D., Moens P. B. Two-dimensional protein patterns during growth and sporulation in Saccharomyces cerevisiae. J Bacteriol. 1979 Apr;138(1):60–69. doi: 10.1128/jb.138.1.60-69.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weik R. R., John D. T. Agitated mass cultivation of Naegleria fowleri. J Parasitol. 1977 Oct;63(5):868–871. [PubMed] [Google Scholar]
- Weik R. R., John D. T. Macromolecular composition and nuclear number during growth of Naegleria fowleri. J Parasitol. 1978 Aug;64(4):746–747. [PubMed] [Google Scholar]