Abstract
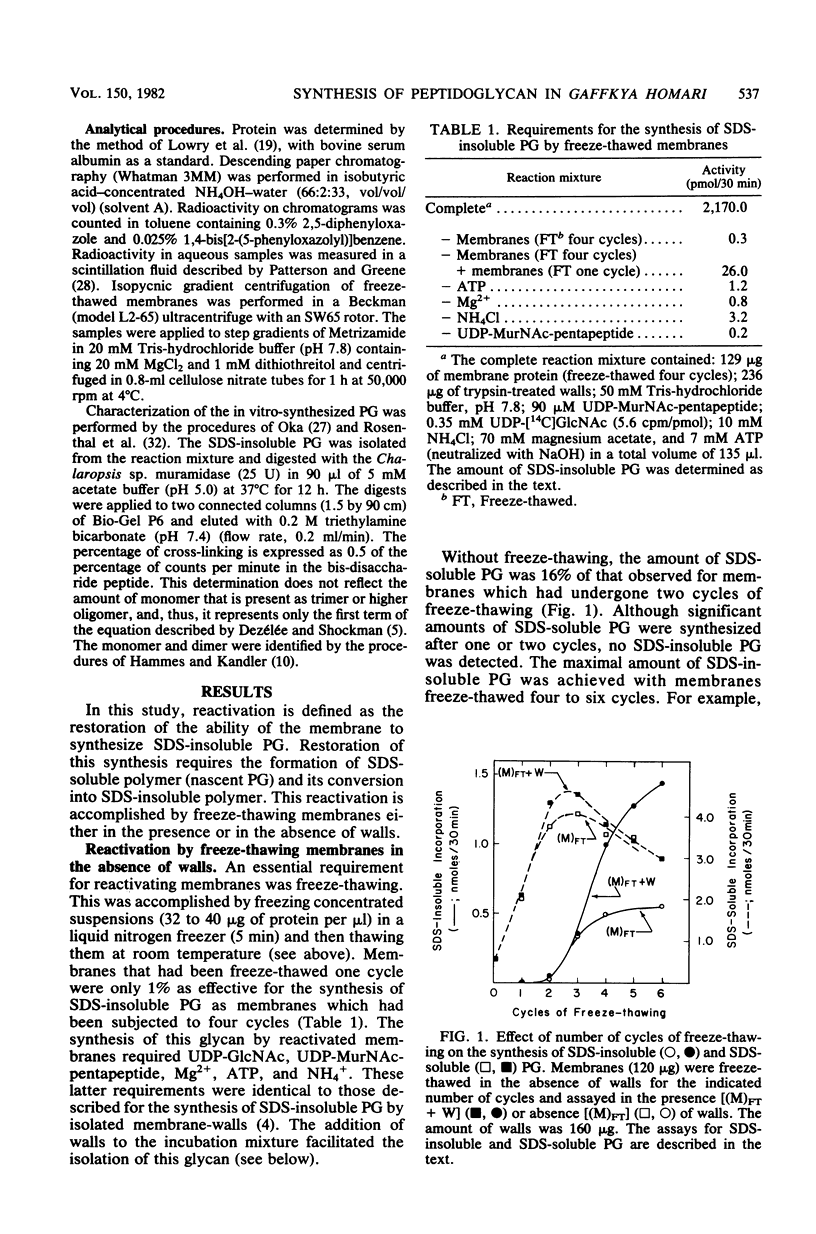
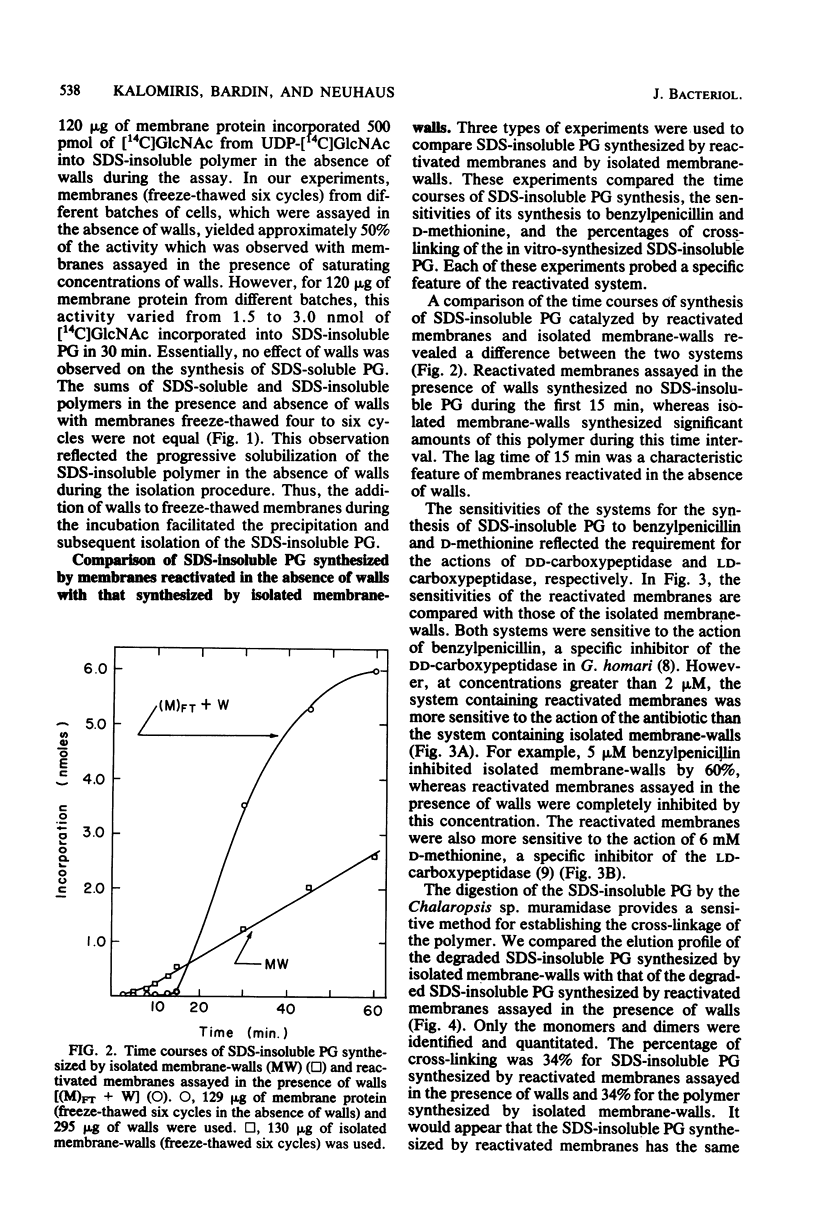
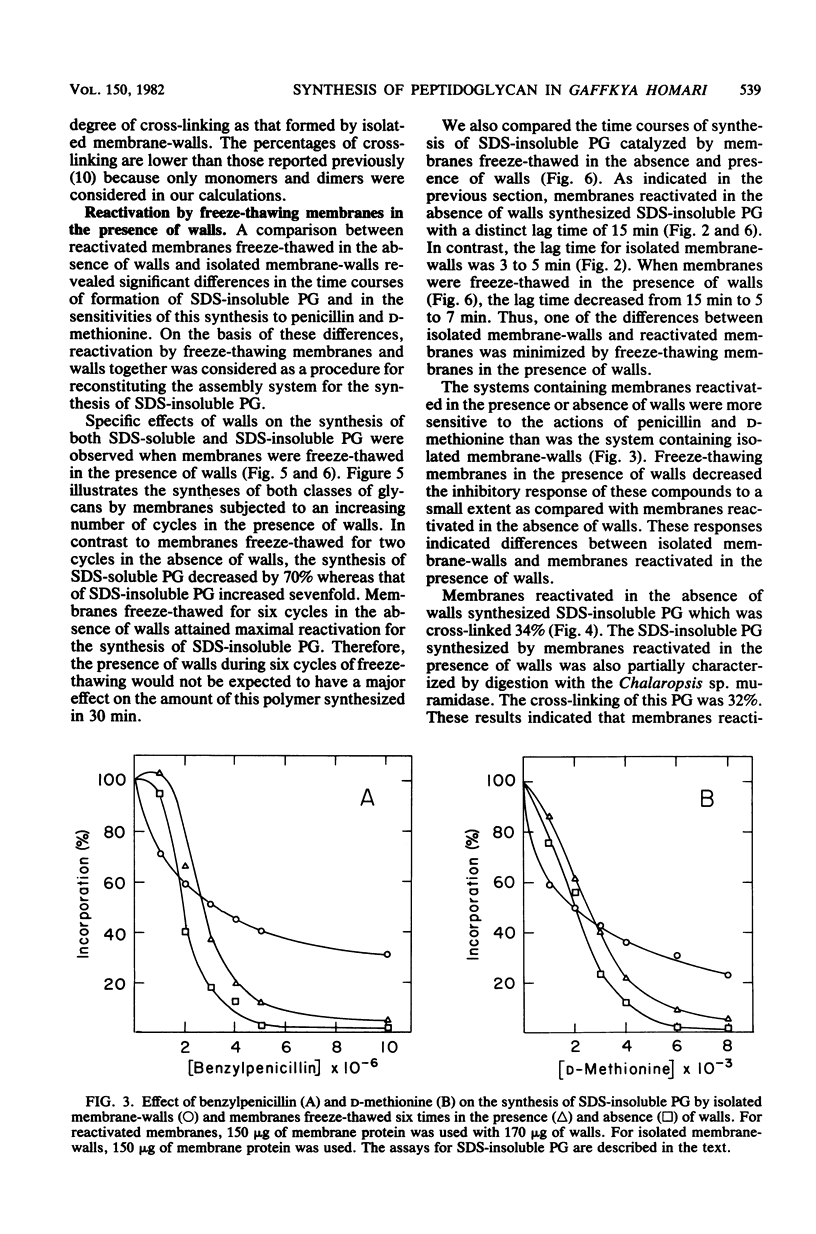
The reactivation of membranes from Gaffkya homari for the synthesis of sodium dodecyl sulfate-insoluble peptidoglycan (SDS-insoluble PG) was achieved by successive cycles of freeze-thawing (− 196 versus 25°C). The presence of G. homari walls during this process affected the synthesis of both SDS-soluble (nascent) and SDS-insoluble PG. At two cycles the synthesis of SDS-soluble PG decreased by 70%, whereas that of SDS-insoluble PG increased sevenfold when compared with membranes reactivated in the absence of walls but assayed in the presence of walls. Moreover, at six cycles the lag time for the synthesis of SDS-insoluble PG decreased from 15 min to 5 to 7 min. Walls from G. homari could not be replaced with walls from Bacillus megaterium or cellulose. In addition to these effects, the presence of walls from G. homari or B. megaterium or of cellulose during the incubation of membranes freeze-thawed in the absence of walls increased twofold the amount of SDS-insoluble PG. Reactivated membranes showed greater sensitivities to penicillin (an inhibitor of dd-carboxypeptidase) and d-methionine (an inhibitor of ld-carboxypeptidase) than did isolated membrane-walls. The percentage of cross-linking of the SDS-insoluble PG synthesized by the reactivated system was 34%, a value similar to that observed for the polymer synthesized by isolated membrane-walls. Freeze-thawing membranes and walls together gave a complex with a density different from that of either membranes or walls. Thus, the assembly system for the synthesis and processing of PG was reconstituted in a complex of membranes and walls prepared from the isolated components. Whether this complex has the exact interrelationship between membrane and wall found in the organism has not been established.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aithal H. N., Kalra V. K., Brodie A. F. Alteration of Mycobacterium phlei membrane structure by freezing and thawing: reversal by heating. Arch Biochem Biophys. 1975 May;168(1):122–132. doi: 10.1016/0003-9861(75)90235-0. [DOI] [PubMed] [Google Scholar]
- Altendorf K. H., Staehelin L. A. Orientation of membrane vesicles from Escherichia coli as detected by freeze-cleave electron microscopy. J Bacteriol. 1974 Feb;117(2):888–899. doi: 10.1128/jb.117.2.888-899.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belous A. M., Bondarenko V. A., Bondarenko T. P. Vliianie zamorazhivaniia-ottaivaniia na lipidnyi sostav mitokhondrii. Biokhimiia. 1978 Dec;43(12):2175–2182. [PubMed] [Google Scholar]
- Carpenter C. V., Goyer S., Neuhaus F. C. Steric effects on penicillin-sensitive peptidoglycan synthesis in a membrane-wall system Gaffkya homari. Biochemistry. 1976 Jul 13;15(14):3146–3152. doi: 10.1021/bi00659a031. [DOI] [PubMed] [Google Scholar]
- Dezélée P., Shockman G. D. Studies of the formation of peptide cross-links in the cell wall peptidoglycan of Streptococcus faecalis. J Biol Chem. 1975 Sep 10;250(17):6806–6816. [PubMed] [Google Scholar]
- Gottesfeld J. M. Methods for fractionation of chromatin into transcriptionally active and inactive segments. Methods Cell Biol. 1977;16:421–436. doi: 10.1016/s0091-679x(08)60117-x. [DOI] [PubMed] [Google Scholar]
- Hammes W. P. Biosynthesis of peptidoglycan in Gaffkya homari. The mode of action of penicillin G and mecillinam. Eur J Biochem. 1976 Nov 1;70(1):107–113. doi: 10.1111/j.1432-1033.1976.tb10961.x. [DOI] [PubMed] [Google Scholar]
- Hammes W. P., Kandler O. Biosynthesis of peptidoglycan in Gaffkya homari. The incorporation of peptidoglycan into the cell wall and the direction of transpeptidation. Eur J Biochem. 1976 Nov 1;70(1):97–106. doi: 10.1111/j.1432-1033.1976.tb10960.x. [DOI] [PubMed] [Google Scholar]
- Hammes W. P., Neuhaus F. C. Biosynthesis of peptidoglycan in Gaffkya homari: role of the peptide subunit of uridine diphosphate-N-acetylmuramyl-pentapeptide. J Bacteriol. 1974 Oct;120(1):210–218. doi: 10.1128/jb.120.1.210-218.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes W. P., Neuhaus F. C. On the specificity of phospho-N-acetylmuramyl-pentapeptide translocase. The peptide subunit of uridine diphosphate-N-actylmuramyl-pentapeptide. J Biol Chem. 1974 May 25;249(10):3140–3150. [PubMed] [Google Scholar]
- Hammes W. P., Seidel H. The activities in vitro of DD-carboxypeptidase and LD-carboxypeptidase of Gaffkya homari during biosynthesis of peptidoglycan. Eur J Biochem. 1978 Mar;84(1):141–147. doi: 10.1111/j.1432-1033.1978.tb12150.x. [DOI] [PubMed] [Google Scholar]
- Hammes W. P. The LD-carboxypeptidase activity in Gaffkya homari. The target of the action of D-amino acids or glycine on the formation of wall-bound peptidoglycan. Eur J Biochem. 1978 Nov 15;91(2):501–507. doi: 10.1111/j.1432-1033.1978.tb12703.x. [DOI] [PubMed] [Google Scholar]
- Hancock I. C., Baddiley J. Solubilisation of a teichoic acid-synthesising system from the membrane of Bacillus licheniformis by freezing and thawing. FEBS Lett. 1973 Aug 1;34(1):15–18. doi: 10.1016/0014-5793(73)80692-1. [DOI] [PubMed] [Google Scholar]
- Kanegasaki S., Wright A. Mechanism of polymerization of the Salmonella O-antigen: utilization of lipid-linked intermediates. Proc Natl Acad Sci U S A. 1970 Oct;67(2):951–958. doi: 10.1073/pnas.67.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Kelly K. F., Evans J. B. Deoxyribonucleic acid homology among strains of the lobster pathogen 'Gaffkya homari' and Aerococcus viridans. J Gen Microbiol. 1974 Mar;81(1):257–260. doi: 10.1099/00221287-81-1-257. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lepock J. R., Morse P. D., 2nd, Keith A. D., Kruuv J. Freeze-thaw damage in isolated lobster sarcoplasmic reticulum membranes: a model system for membrane damage. Cryobiology. 1978 Dec;15(6):643–653. doi: 10.1016/0011-2240(78)90089-5. [DOI] [PubMed] [Google Scholar]
- Mazur P. Cryobiology: the freezing of biological systems. Science. 1970 May 22;168(3934):939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Role of the penicillin-sensitive transpeptidation reaction in attachment of newly synthesized peptidoglycan to cell walls of Micrococcus luteus. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3355–3359. doi: 10.1073/pnas.69.11.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Studies on the elongation of bacterial cell wall peptidoglycan and its inhibition by penicillin. Ann N Y Acad Sci. 1974 May 10;235(0):326–347. doi: 10.1111/j.1749-6632.1974.tb43275.x. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Nuchamowitz Y. Biosynthesis of peptidoglycan in Pseudomonas aeruginosa. 1. The incorporation of peptidoglycan into the cell wall. Eur J Biochem. 1979 Mar;94(2):541–548. doi: 10.1111/j.1432-1033.1979.tb12923.x. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Sharon N. Biosynthesis of peptidoglycan by a cell wall preparation of Staphylococcus aureus and its inhibition by penicillin. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1909–1917. doi: 10.1016/0006-291x(72)90069-1. [DOI] [PubMed] [Google Scholar]
- Neuhaus F. C., Tobin C. E., Ahlgren J. A. Membrane-wall interrelationship in Gaffkya homari: sulfhydryl sensitivity and heat lability of nascent peptidoglycan incorporation into walls. J Bacteriol. 1980 Jul;143(1):112–119. doi: 10.1128/jb.143.1.112-119.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T. Mode of action of penicillins in vivo and in vitro in Bacillus megaterium. Antimicrob Agents Chemother. 1976 Oct;10(4):579–591. doi: 10.1128/aac.10.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E. Peptidoglycan synthesis in bacilli. II. Characteristics of protoplast membrane preparations. Biochim Biophys Acta. 1971 May 18;237(2):255–272. doi: 10.1016/0304-4165(71)90316-3. [DOI] [PubMed] [Google Scholar]
- Rickwood D., Hell A., Birnie G. D. Isopycnic centrifugation of sheared chromatin in metrizamide gradients. FEBS Lett. 1973 Jul 1;33(2):221–224. doi: 10.1016/0014-5793(73)80197-8. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. S., Wright R. M., Sinha R. K. Extent of peptide cross-linking in the peptidoglycan of Neisseria gonorrhoeae. Infect Immun. 1980 Jun;28(3):867–875. doi: 10.1128/iai.28.3.867-875.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R. Isolation of cell walls from Gram-positive bacteria. Methods Enzymol. 1974;31:653–667. doi: 10.1016/0076-6879(74)31071-3. [DOI] [PubMed] [Google Scholar]
- Struve W. G., Sinha R. K., Neuhaus F. C. On the initial stage in peptidoglycan synthesis. Phospho-N-acetylmuramyl-pentapeptide translocase (uridine monophosphate). Biochemistry. 1966 Jan;5(1):82–93. doi: 10.1021/bi00865a012. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Konisky J. In vitro depolarization of Escherichia coli membrane vesicles by colicin Ia. J Biol Chem. 1978 Nov 10;253(21):7731–7737. [PubMed] [Google Scholar]
- Ward J. B., Perkins H. R. Peptidoglycan biosynthesis by preparations from Bacillus licheniformis: cross-linking of newly synthesized chains to preformed cell wall. Biochem J. 1974 Jun;139(3):781–784. doi: 10.1042/bj1390781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B. The synthesis of peptidoglycan in an autolysin-deficient mutant of Bacillus licheniformis N.C.T.C. 6346 and the effect of beta-lactam antibiotics, bacitracin and vancomycin. Biochem J. 1974 Jul;141(1):227–241. doi: 10.1042/bj1410227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman A. M., Pendyala L. Permeability changes in membranes of Neurospora crassa after freezing and thawing. Cryobiology. 1979 Apr;16(2):184–195. doi: 10.1016/0011-2240(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Wientjes F. B., van 't Piet J., Nanninga N. Formation of inside-out vesicles of Bacillus licheniformis. Dependence on buffer composition and lysis procedure. Biochim Biophys Acta. 1979 May 17;553(2):213–223. doi: 10.1016/0005-2736(79)90226-8. [DOI] [PubMed] [Google Scholar]



