Abstract
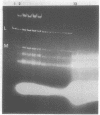
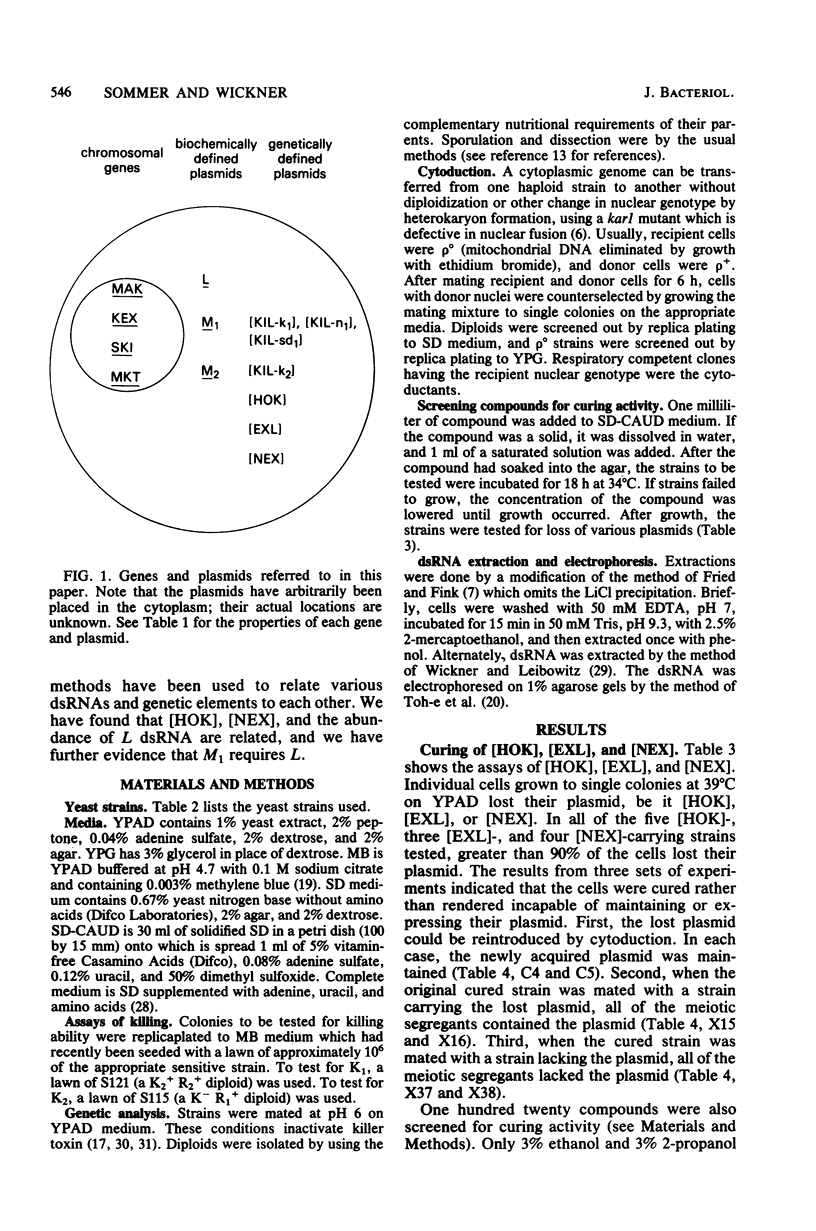
We describe two sets of plasmid-plasmid interactions in the yeast Saccharomyces cerevisiae. [HOK], [EXL], [NEX], and [KIL-k1] are genetically defined plasmids, and M1 and L are biochemically defined double-stranded RNA plasmids. We show that (i) [HOK], [NEX], and the abundance of L are related, and (ii) under submaximal curing conditions, all colonies retaining M1 also retain L. There are three pieces of evidence that either [NEX] required [HOK] for replication or [NEX] and [HOK] are on the same plasmid. The evidence is as follows. (i) The great majority of strains containing [HOK] also contain [NEX]. However, two [HOK] [NEX-o] strains do exist. (ii) Growth at 39 degrees C or growth at 34 degrees C with 3% ethanol or 2-propanol cures [HOK] and [NEX]. In a [HOK] [NEX] strain, the two plasmids are always co-cured. (iii) [HOK] and [NEX] are both maintained in mak4, mak6, and mak27 strains (mak = maintenance of [KIL-k1]), but not in mak3, mak10, and pet18 strains. Strains containing [HOK] and [NEX] have about fourfold more L double-stranded RNA than their isochromosomal, cured derivatives. In addition, a cytoductant which has acquired [HOK] and [NEX] has fourfold more L than its parent. These results are consistent with either [HOK] being a form of L or [HOK] increasing the copy number of L. Using a K1 killer strain in which L, as well as M1, could be cured by growth at 38 degrees C, we examined the distribution of loss of M1 and L under conditions giving 98% M-o colonies and at least 50% L-o colonies. No M1L-o colonies were observed, supporting the previous suggestion by others that M1 requires L.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan E. A., Herring A. J., Mitchell D. J. Preliminary characterization of two species of dsRNA in yeast and their relationship to the "killer" character. Nature. 1973 Sep 14;245(5420):81–86. doi: 10.1038/245081b0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Sturgeon J. A., Tipper D. J. Encapsidation of yeast killer double-stranded ribonucleic acids: dependence of M on L. J Bacteriol. 1980 Jul;143(1):463–470. doi: 10.1128/jb.143.1.463-470.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J. A., Brennan V. E. Yeast viral double-stranded RNAs have heterogeneous 3' termini. Cell. 1980 Apr;19(4):923–933. doi: 10.1016/0092-8674(80)90084-7. [DOI] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H., Wickner R. B. Spermidine or spermine requirement for killer double-stranded RNA plasmid replication in yeast. J Biol Chem. 1978 Aug 10;253(15):5225–5227. [PubMed] [Google Scholar]
- Conde J., Fink G. R. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried H. M., Fink G. R. Electron microscopic heteroduplex analysis of "killer" double-stranded RNA species from yeast. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4224–4228. doi: 10.1073/pnas.75.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Bevan E. A. Virus-like particles associated with the double-stranded RNA species found in killer and sensitive strains of the yeast Saccharomyces cerevisiae. J Gen Virol. 1974 Mar;22(3):387–394. doi: 10.1099/0022-1317-22-3-387. [DOI] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Ishii K., Hashimoto-Gotoh T., Matsubara K. Random replication and random assortment model for plasmid incompatibility in bacteria. Plasmid. 1978 Sep;1(4):435–445. doi: 10.1016/0147-619x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Leibowitz M. J., Wickner R. B. Pet18: a chromosomal gene required for cell growth and for the maintenance of mitochondrial DNA and the killer plasmid of yeast. Mol Gen Genet. 1978 Oct 4;165(2):115–121. doi: 10.1007/BF00269899. [DOI] [PubMed] [Google Scholar]
- Mitchell D. J., Herring A. J., Bevan E. A. The genetic control of DS-RNA virus-like particles associated with Saccharomyces cerevisiae killer yeast. Heredity (Edinb) 1976 Aug;37(1):129–134. doi: 10.1038/hdy.1976.71. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in yeast. Methods Cell Biol. 1975;11:221–233. doi: 10.1016/s0091-679x(08)60325-8. [DOI] [PubMed] [Google Scholar]
- Naumova G. I., Naumova T. I. Sravitel'naia genetika drozhzhei. Soobshchenie XIII. Sravitel'noe izuchenie sakharomitsetov-ubiits iz razlichnykh kollektsii. Genetika. 1973 Nov;9(11):140–145. [PubMed] [Google Scholar]
- Novick R. P., Hoppensteadt F. C. On plasmid incompatibility. Plasmid. 1978 Sep;1(4):421–434. doi: 10.1016/0147-619x(78)90001-x. [DOI] [PubMed] [Google Scholar]
- Palfree R. G., Bussey H. Yeast killer toxin: purification and characterisation of the protein toxin from Saccharomyces cerevisiae. Eur J Biochem. 1979 Feb 1;93(3):487–493. doi: 10.1111/j.1432-1033.1979.tb12847.x. [DOI] [PubMed] [Google Scholar]
- Philliskirk G., Young T. W. The occurrence of killer character in yeasts of various genera. Antonie Van Leeuwenhoek. 1975;41(2):147–151. doi: 10.1007/BF02565046. [DOI] [PubMed] [Google Scholar]
- SHERMAN F. The effects of elevated temperatures on yeast. II. Induction of respiratory-deficient mutants. J Cell Comp Physiol. 1959 Aug;54:37–52. doi: 10.1002/jcp.1030540106. [DOI] [PubMed] [Google Scholar]
- Somers J. M., Bevan E. A. The inheritance of the killer character in yeast. Genet Res. 1969 Feb;13(1):71–83. doi: 10.1017/s0016672300002743. [DOI] [PubMed] [Google Scholar]
- Toh-E A., Guerry P., Wickner R. B. Chromosomal superkiller mutants of Saccharomyces cerevisiae. J Bacteriol. 1978 Dec;136(3):1002–1007. doi: 10.1128/jb.136.3.1002-1007.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-E A., Wickner R. B. "Superkiller" mutations suppress chromosomal mutations affecting double-stranded RNA killer plasmid replication in saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jan;77(1):527–530. doi: 10.1073/pnas.77.1.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-E A., Wickner R. B. A mutant killer plasmid whose replication depends on a chromosomal "superkiller" mutation. Genetics. 1979 Apr;91(4):673–682. doi: 10.1093/genetics/91.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M., Katterman F., Fink G. R. Yeast killer mutants with altered double-stranded ribonucleic acid. J Bacteriol. 1974 Feb;117(2):681–686. doi: 10.1128/jb.117.2.681-686.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. "Killer character" of Saccharomyces cerevisiae: curing by growth at elevated temperature. J Bacteriol. 1974 Mar;117(3):1356–1357. doi: 10.1128/jb.117.3.1356-1357.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Chromosomal and nonchromosomal mutations affecting the "killer character" of Saccharomyces cerevisiae. Genetics. 1974 Mar;76(3):423–432. doi: 10.1093/genetics/76.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Two chromosomal genes required for killing expression in killer strains of Saccharomyces cerevisiae. Genetics. 1976 Mar 25;82(3):429–442. doi: 10.1093/genetics/82.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Plasmids controlled exclusion of the K2 killer double-stranded RNA plasmid of yeast. Cell. 1980 Aug;21(1):217–226. doi: 10.1016/0092-8674(80)90129-4. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. The killer double-stranded RNA plasmids of yeast. Plasmid. 1979 Jul;2(3):303–322. doi: 10.1016/0147-619x(79)90015-5. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Twenty-six chromosomal genes needed to maintain the killer double-stranded RNA plasmid of Saccharomyces cerevisiae. Genetics. 1978 Mar;88(3):419–425. doi: 10.1093/genetics/88.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. R., Bevan E. A. Studies on the nature of the killer factor produced by Saccharomyces cerevisiae. J Gen Microbiol. 1968 Apr;51(1):115–126. doi: 10.1099/00221287-51-1-115. [DOI] [PubMed] [Google Scholar]
- Young T. W., Yagiu M. A comparison of the killer character in different yeasts and its classification. Antonie Van Leeuwenhoek. 1978;44(1):59–77. doi: 10.1007/BF00400077. [DOI] [PubMed] [Google Scholar]