Abstract
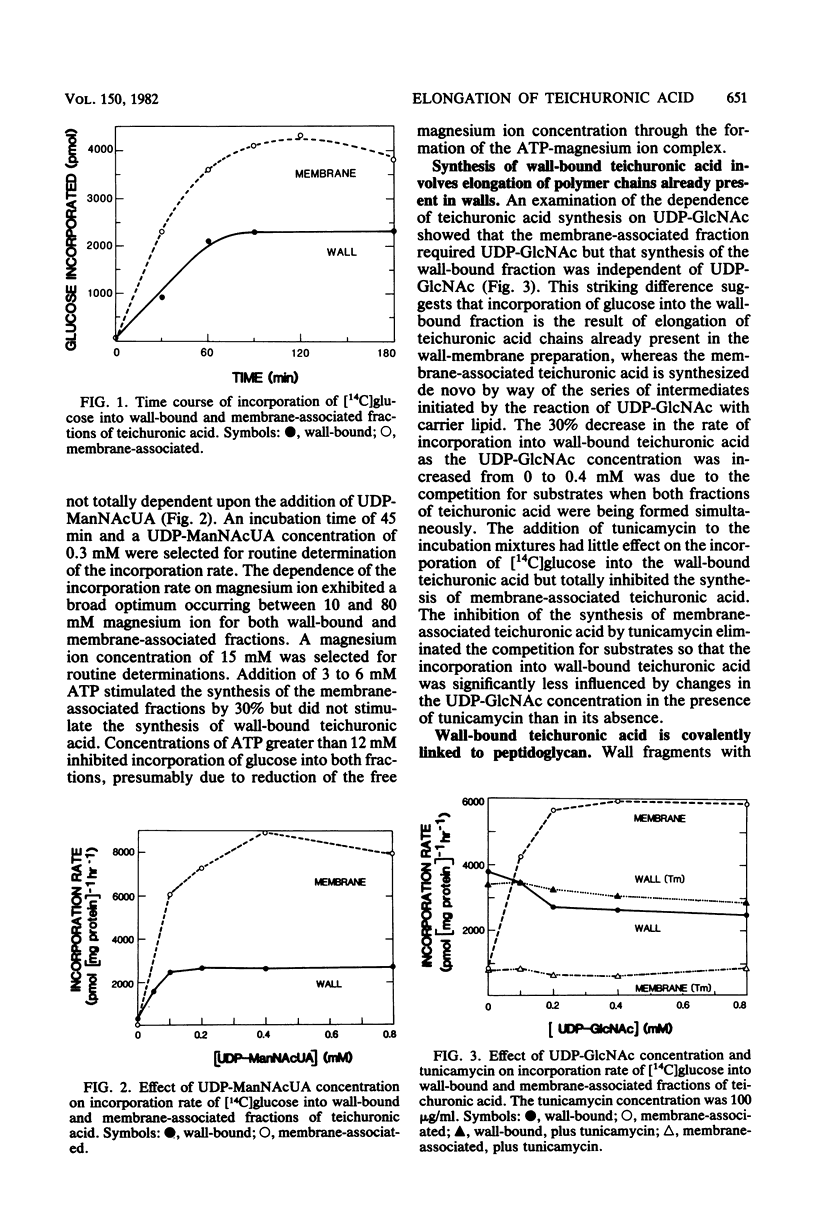
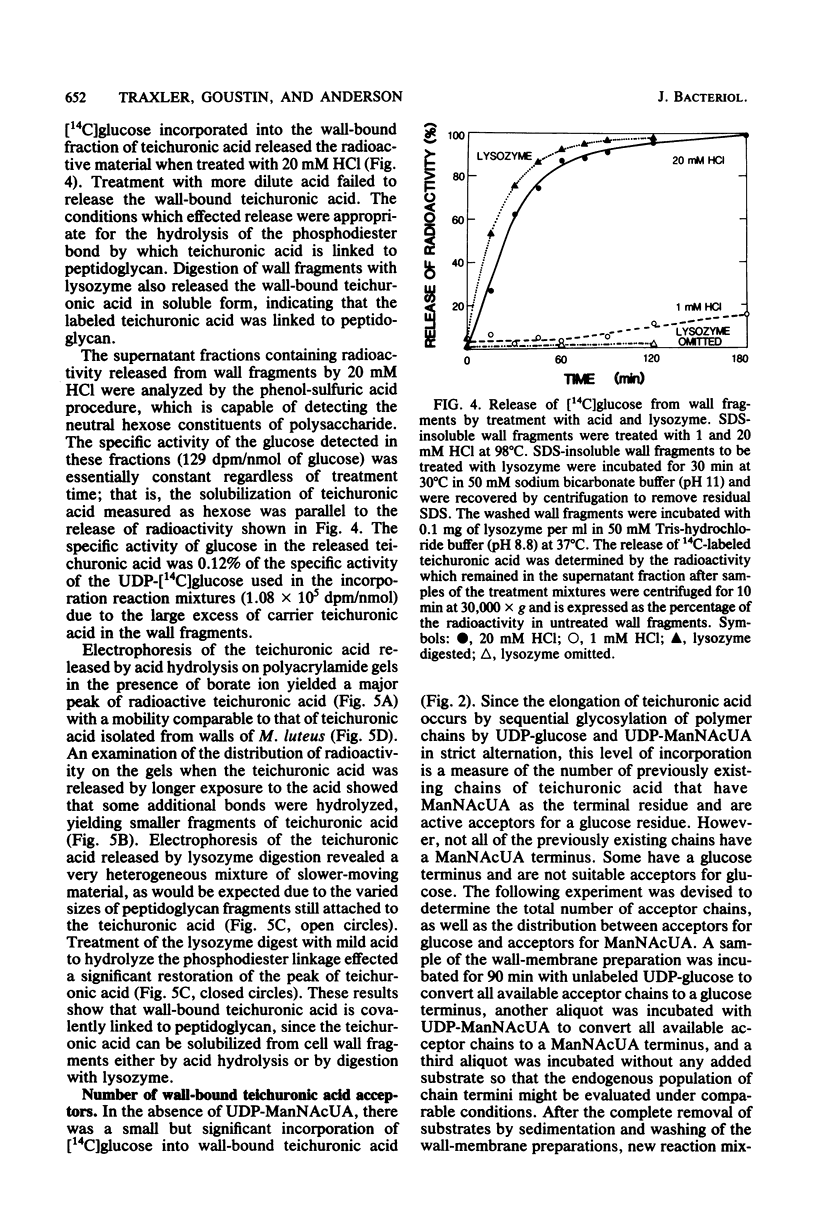
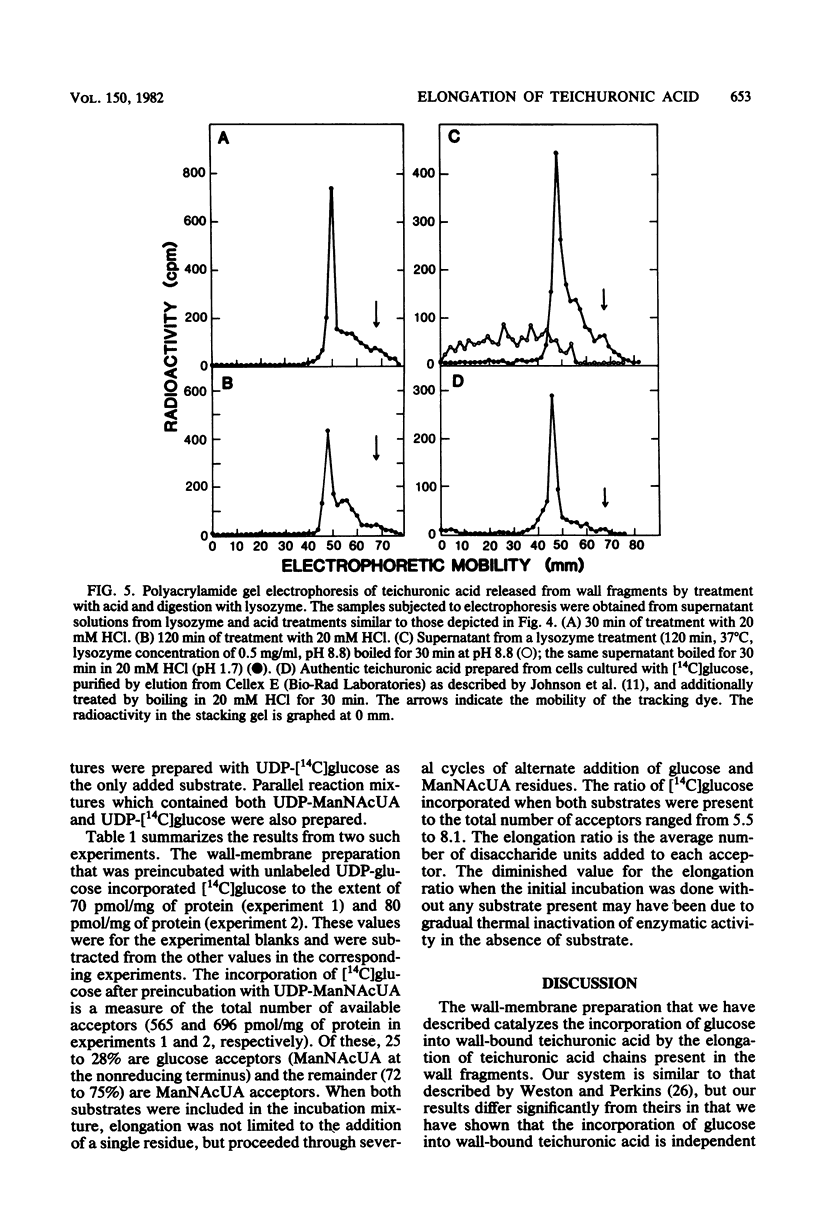
A wall-plus-membrane preparation from Micrococcus luteus catalyzes the incorporation of [14C]glucose from UDP-[14C]glucose, into two fractions of teichuronic acid, which is the cell wall polysaccharide consisting of alternating residues of glucose and N-acetylmannosaminuronic acid (ManNAcUA). Membrane-associated teichuronic acid was extracted from the wall-membrane fraction of reaction mixtures by sodium dodecyl sulfate. The synthesis of membrane-associated teichuronic acid required UDP-glucose, UDP-ManNAcUA, and UDP-N-acetylglucosamine and was inhibited by tunicamycin. Glucose incorporated into wall-bound teichuronic acid remained in wall fragments after extraction with sodium dodecyl sulfate, and its incorporation required UDP-glucose and UDP-ManNAcUA (but not UDP-N-acetylglucosamine) and was insensitive to tunicamycin. Radioactive material incorporated into wall-bound teichuronic acid could be released by treatment with mild acid or by digestion with lysozyme, indicating that the wall-bound teichuronic acid was covalently linked to peptidoglycan. There were about 600 pmol of wall-bound teichuronic acid acceptor sites for glucose per mg of protein as measured in incorporation reaction mixtures lacking UDP-ManNAcUA. In the presence of both UDP-glucose and UDP-ManNAcUA, elongation of teichuronic acid acceptor sites occurred, with the addition of six to eight disaccharide units to each acceptor site.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. S., Page R. L. Biosynthesis of the polysaccharide of Micrococcus lysodeikticus cell walls. II. Identification of uridine diphospho-N-acetyl-D-mannosaminuronic acid as required substrate. J Biol Chem. 1972 Apr 25;247(8):2480–2485. [PubMed] [Google Scholar]
- Anker H. S. A solubilizable acrylamide gel for electrophoresis. FEBS Lett. 1970 Apr 16;7(3):293–293. doi: 10.1016/0014-5793(70)80185-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Campbell J. N., Leyh-Bouille M., Ghuysen J. M. Characterization of the Micrococcus lysodeikticus type of peptidoglycan in walls of other Micrococcaceae. Biochemistry. 1969 Jan;8(1):193–200. doi: 10.1021/bi00829a028. [DOI] [PubMed] [Google Scholar]
- Din N. U., Jeanloz R. W. The chemical structure of a fragment of Micrococcus lysodeikticus cell-wall. Carbohydr Res. 1976 Apr;47(2):245–260. doi: 10.1016/s0008-6215(00)84190-7. [DOI] [PubMed] [Google Scholar]
- Hase S., Matsushima Y. Structural studies on a glucose-containing polysaccharide obtained from cell walls of Micrococcus lysodeikticus. 3. Determination of the structure. J Biochem. 1972 Nov;72(5):1117–1128. doi: 10.1093/oxfordjournals.jbchem.a129999. [DOI] [PubMed] [Google Scholar]
- Hase S., Matsushima Y. The structure of the branching point between acidic polysaccharide and peptidoglycan in Micrococcus lysodeikticus cell wall. J Biochem. 1977 May;81(5):1181–1186. [PubMed] [Google Scholar]
- Heifetz A., Keenan R. W., Elbein A. D. Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry. 1979 May 29;18(11):2186–2192. doi: 10.1021/bi00578a008. [DOI] [PubMed] [Google Scholar]
- Ichihara N., Ishimoto N., Ito E. Enzymatic formation of uridine diphosphate N-acetyl-D-mannosaminuronic acid. FEBS Lett. 1974 Feb 1;39(1):46–48. doi: 10.1016/0014-5793(74)80013-x. [DOI] [PubMed] [Google Scholar]
- Imanaga Y., Park J. T. Studies on the cell walls of Micrococcus lysodeikticus. Fractionation of the nondialyzable components from a lysozyme digest of cell walls. Biochemistry. 1972 Oct 10;11(21):4006–4012. doi: 10.1021/bi00771a026. [DOI] [PubMed] [Google Scholar]
- Johnson S. D., Lacher K. P., Anderson J. S. Carbon-13 nuclear magnetic resonance spectroscopic study of teichuronic acid from Micrococcus luteus cell walls. Comparison of the polysaccharide isolated from cells with that synthesized in vitro. Biochemistry. 1981 Aug 4;20(16):4781–4785. doi: 10.1021/bi00519a039. [DOI] [PubMed] [Google Scholar]
- Keller R. K., Boon D. Y., Crum F. C. N-Acetylglucosamine- 1 -phosphate transferase from hen oviduct: solubilization, characterization, and inhibition by tunicamycin. Biochemistry. 1979 Sep 4;18(18):3946–3952. doi: 10.1021/bi00585a016. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C. Muramic acid phosphate as a component of the mucopeptide of Gram-positive bacteria. J Biol Chem. 1967 Feb 10;242(3):471–476. [PubMed] [Google Scholar]
- McArthur H. A., Hancock I. C., Baddiley J. Attachment of the main chain to the linkage unit in biosynthesis of teichoic acids. J Bacteriol. 1981 Mar;145(3):1222–1231. doi: 10.1128/jb.145.3.1222-1231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Role of the penicillin-sensitive transpeptidation reaction in attachment of newly synthesized peptidoglycan to cell walls of Micrococcus luteus. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3355–3359. doi: 10.1073/pnas.69.11.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- PERKINS H. R. A polymer containing glucose and aminohexuronic acid isolated from the cell walls of micrococcus lysodeikticus. Biochem J. 1963 Mar;86:475–483. doi: 10.1042/bj0860475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R. L., Anderson J. S. Biosynthesis of the polysaccharide of micrococcus lysodekticus cells walls. I. Characterization of an in vitro system for polysaccharide biosynthesis. J Biol Chem. 1972 Apr 25;247(8):2471–2479. [PubMed] [Google Scholar]
- Perkins H. R., Thorpe S. J., Brown C. A. Tunicamycin and wall-polymer synthesis in micrococcus luteus and neisseria gonorrhoeae. Biochem Soc Trans. 1980 Apr;8(2):163–164. doi: 10.1042/bst0080163. [DOI] [PubMed] [Google Scholar]
- Rohr T. E., Levy G. N., Stark N. J., Anderson J. S. Initial reactions in biosynthesis of teichuronic acid of Micrococcus lysodeikticus cell walls. J Biol Chem. 1977 May 25;252(10):3460–3465. [PubMed] [Google Scholar]
- Stark N. J., Levy G. N., Rohr T. E., Anderson J. S. Reactions of second stage of biosynthesis of teichuronic acid of Micrococcus lysodeikticus cell walls. J Biol Chem. 1977 May 25;252(10):3466–3472. [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Weston A., Perkins H. R. Biosynthesis of wall-linked teichuronic acid by a wall-plus-membrane preparation from Micrococcus luteus. Effect of antibiotics. FEBS Lett. 1977 Apr 15;76(2):195–198. doi: 10.1016/0014-5793(77)80150-6. [DOI] [PubMed] [Google Scholar]
- Wyke A. W., Ward J. B. Biosynthesis of wall polymers in Bacillus subtilis. J Bacteriol. 1977 Jun;130(3):1055–1063. doi: 10.1128/jb.130.3.1055-1063.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


