Abstract
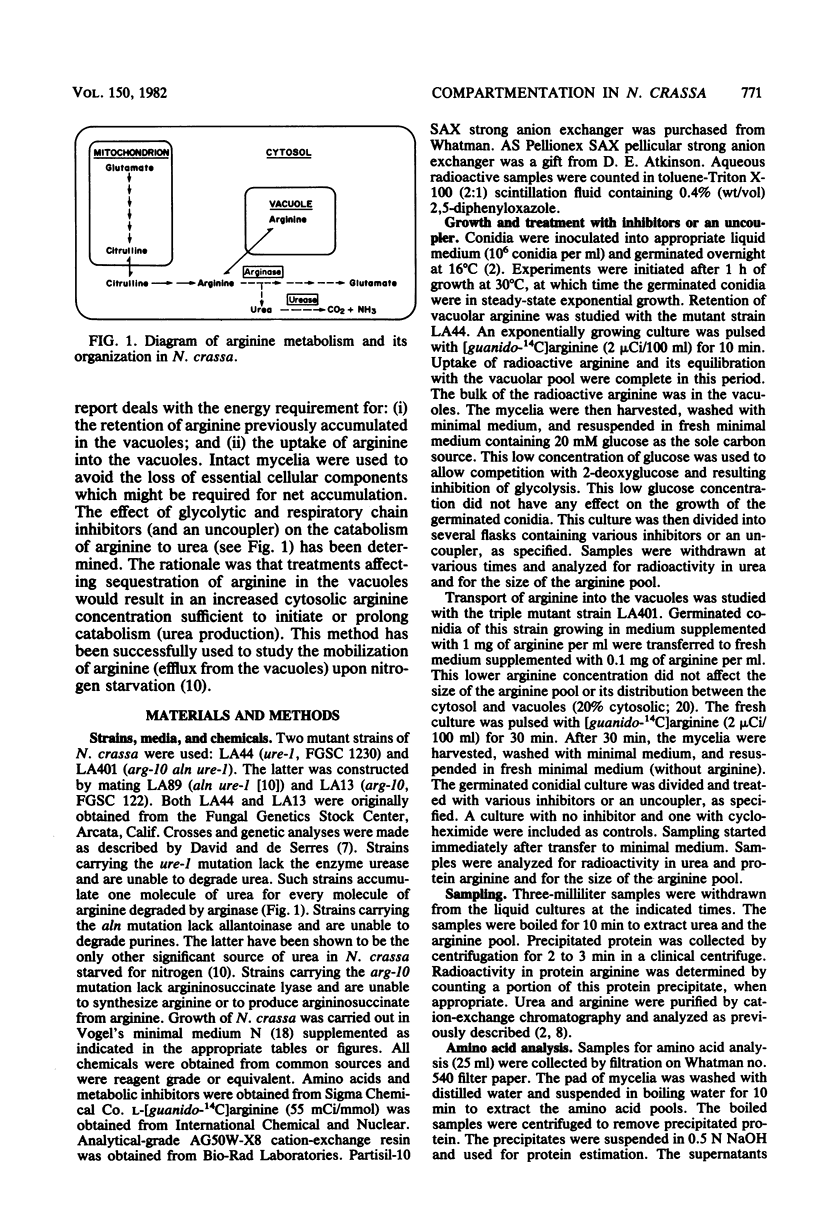
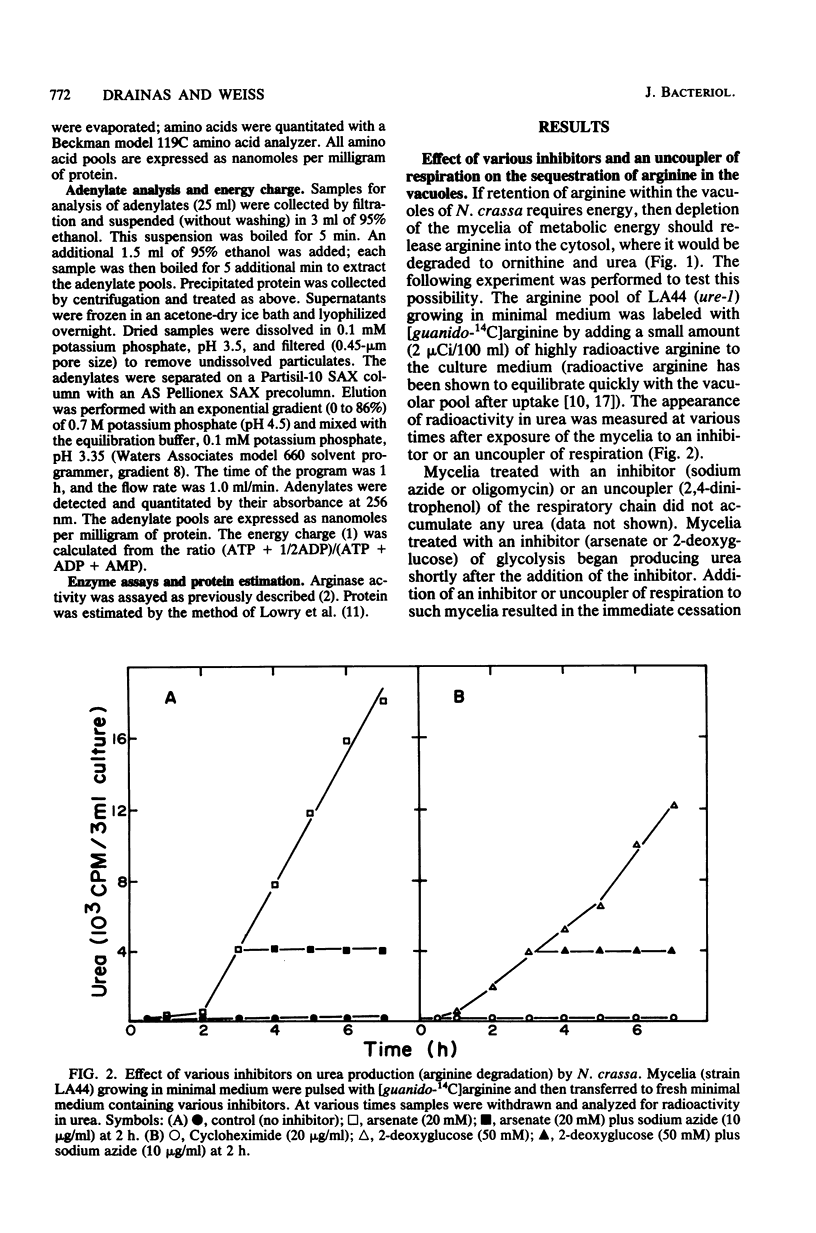
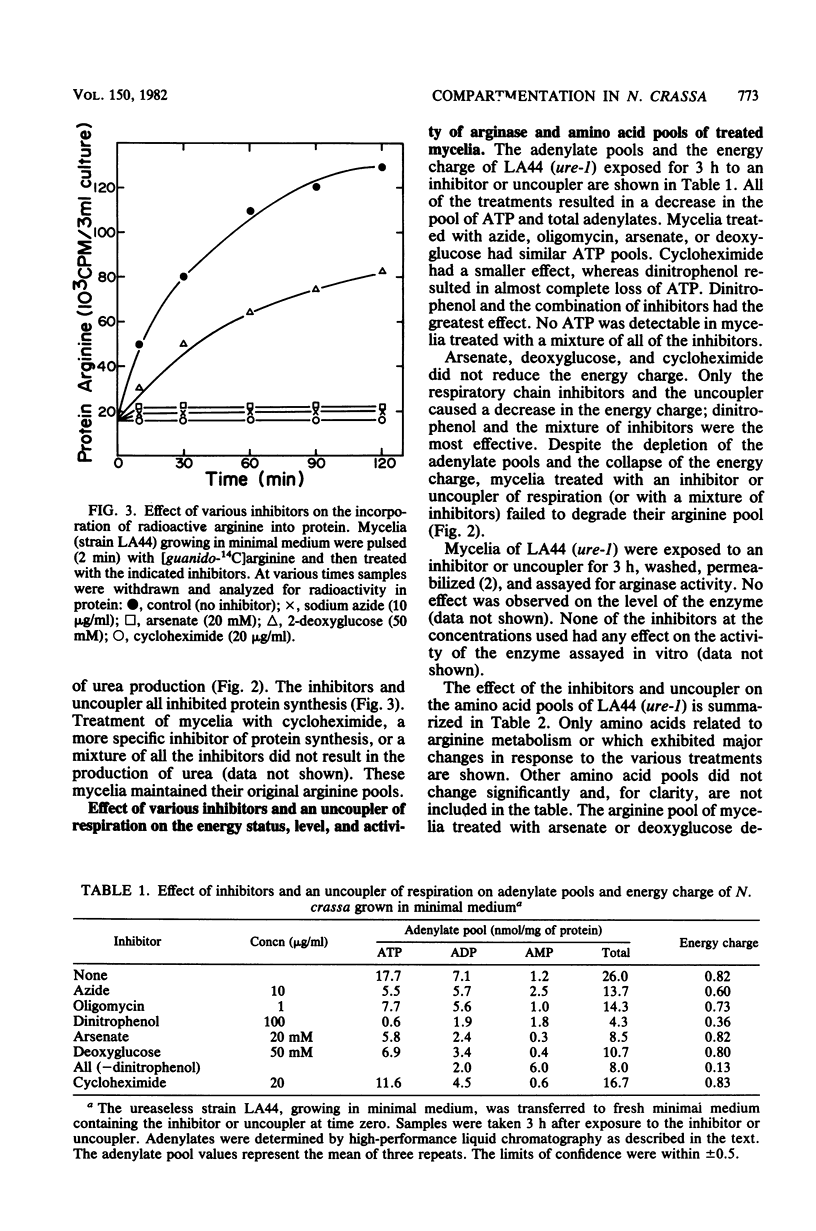
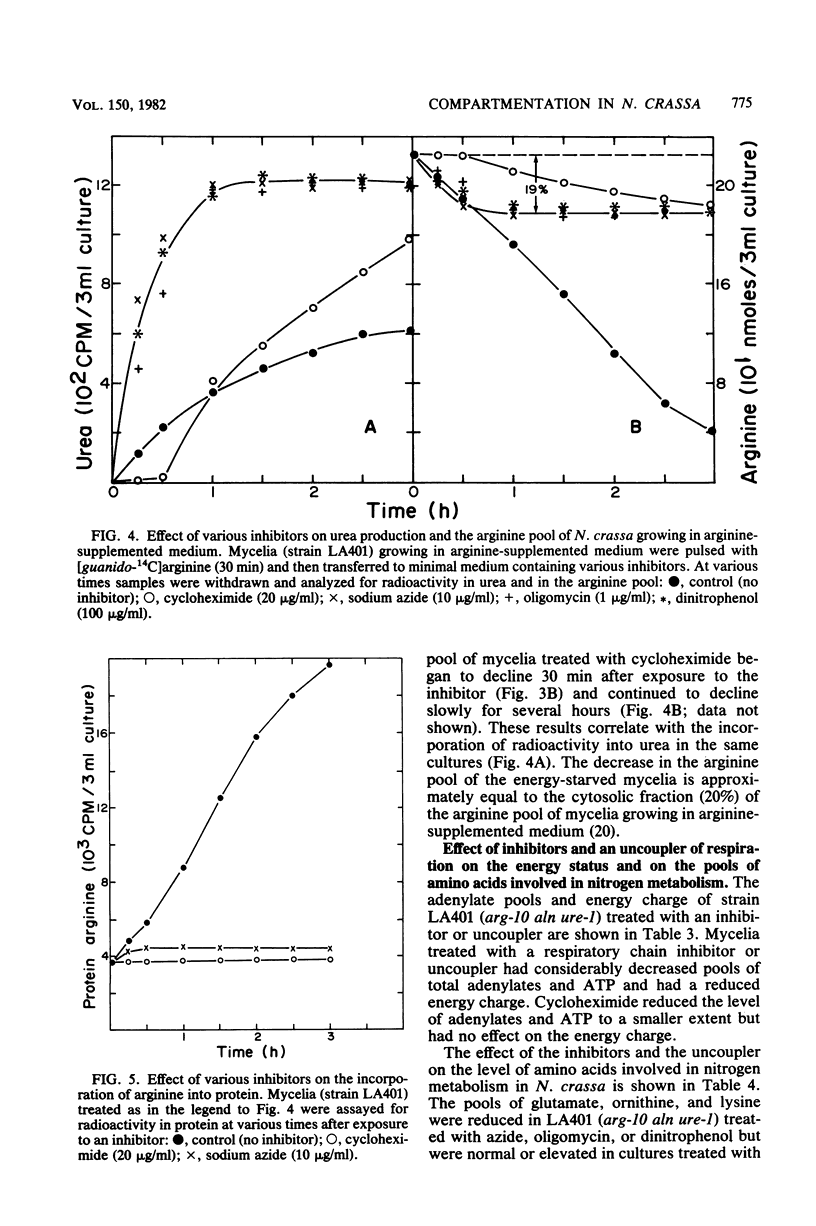
The energy requirements for the uptake and retention of arginine by vacuoles of Neurospora crassa have been studied. Exponentially growing mycelial cultures were treated with inhibitors of respiration or glycolysis or an uncoupler of respiration. Catabolism of arginine was monitored as urea production in urease-less strains. The rationale was that the rate and extent of such catabolism was indicative of the cytosolic arginine concentration. No catabolism was observed in cultures treated with an inhibitor or an uncoupler of respiration, but cultures treated with inhibitors of glycolysis rapidly degraded arginine. These differences could not be accounted for by alterations in the level or activity of arginase. Mycelia growing in arginine-supplemented medium and treated with an inhibitor or uncoupler of respiration degraded an amount of arginine equivalent to the cytosolic fraction of the arginine pool. The inhibitors and the uncoupler of respiration reduced the ATP pool and the energy charge. The inhibitors of glycolysis reduced the ATP pool but did not affect the energy charge. The results suggest that metabolic energy is required for the transport of arginine into the vacuoles but not for its retention. The latter is affected by inhibitors of glycolysis. The form of energy and the nature of the vacuolar transport mechanism(s) are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basabe J. R., Lee C. A., Weiss R. L. Enzyme assays using permeabilized cells of Neurospora. Anal Biochem. 1979 Jan 15;92(2):356–360. doi: 10.1016/0003-2697(79)90670-5. [DOI] [PubMed] [Google Scholar]
- Boller T., Dürr M., Wiemken A. Characterization of a specific transport system for arginine in isolated yeast vacuoles. Eur J Biochem. 1975 May;54(1):81–91. doi: 10.1111/j.1432-1033.1975.tb04116.x. [DOI] [PubMed] [Google Scholar]
- Cramer C. L., Vaughn L. E., Davis R. H. Basic amino acids and inorganic polyphosphates in Neurospora crassa: independent regulation of vacuolar pools. J Bacteriol. 1980 Jun;142(3):945–952. doi: 10.1128/jb.142.3.945-952.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Compartmentation and regulation of fungal metabolism: genetic approaches. Annu Rev Genet. 1975;9:39–65. doi: 10.1146/annurev.ge.09.120175.000351. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Sources of urea in Neurospora. Biochim Biophys Acta. 1970 Aug 14;215(2):412–414. doi: 10.1016/0304-4165(70)90042-5. [DOI] [PubMed] [Google Scholar]
- Drainas C., Weiss R. L. Effect of carbon source on enzymes and metabolites of arginine metabolism in Neurospora. J Bacteriol. 1980 Jan;141(1):205–212. doi: 10.1128/jb.141.1.205-212.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legerton T. L., Weiss R. L. Mobilization of sequestered metabolities into degradative reactions by nutritional stress in Neurospora. J Bacteriol. 1979 Jun;138(3):909–914. doi: 10.1128/jb.138.3.909-914.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E., Heck U., Boller T., Wiemken A., Matile P. Some properties of vacuoles isolated from Neurospora crassa slime variant. Arch Microbiol. 1979 Jan 16;120(1):31–34. doi: 10.1007/BF00413268. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1981 Mar 10;256(5):2079–2082. [PubMed] [Google Scholar]
- Srere P. A., Mosbach K. Metabolic compartmentation: symbiotic, organellar, multienzymic, and microenvironmental. Annu Rev Microbiol. 1974;28(0):61–83. doi: 10.1146/annurev.mi.28.100174.000425. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Compartmentation and control of arginine metabolism in Neurospora. J Bacteriol. 1976 Jun;126(3):1173–1179. doi: 10.1128/jb.126.3.1173-1179.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Control of arginine utilization in Neurospora. J Bacteriol. 1977 Feb;129(2):866–873. doi: 10.1128/jb.129.2.866-873.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]


