Abstract
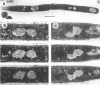
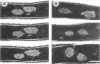
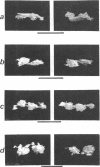
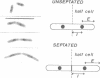
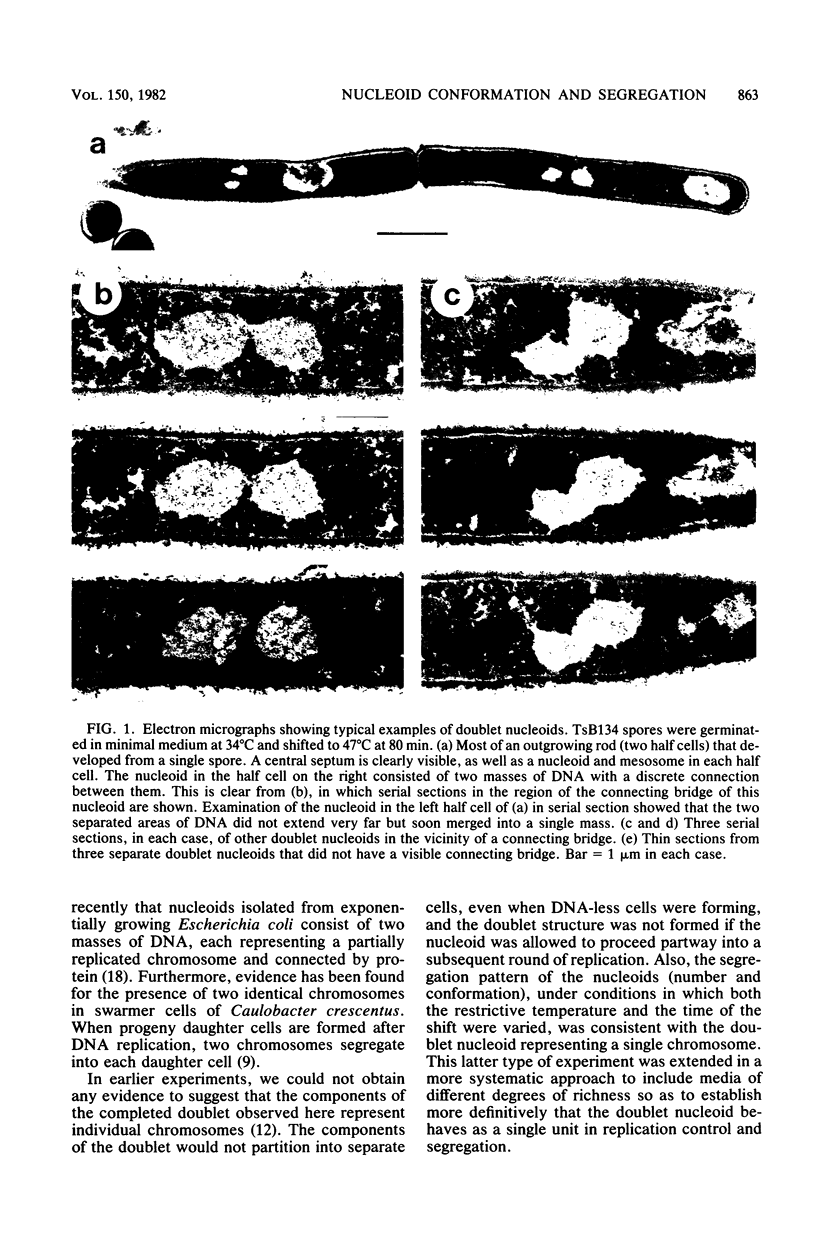
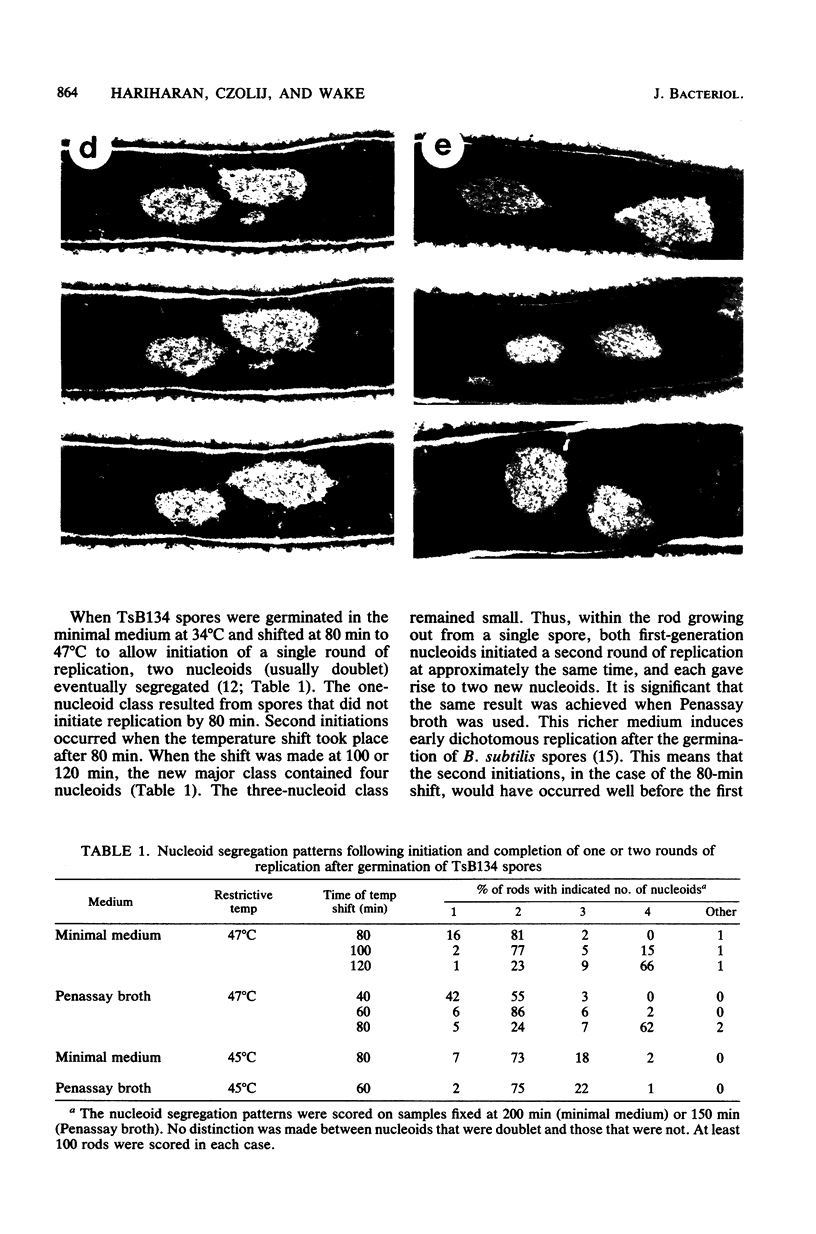
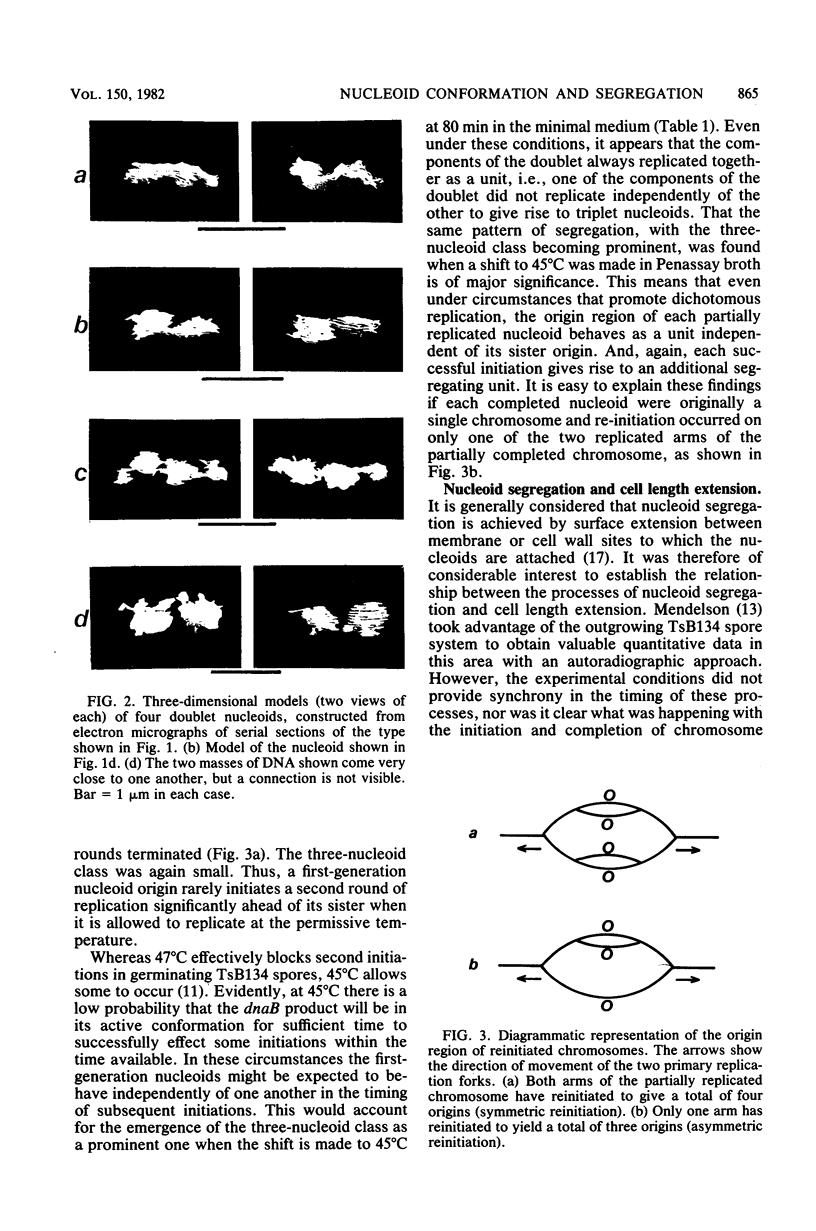
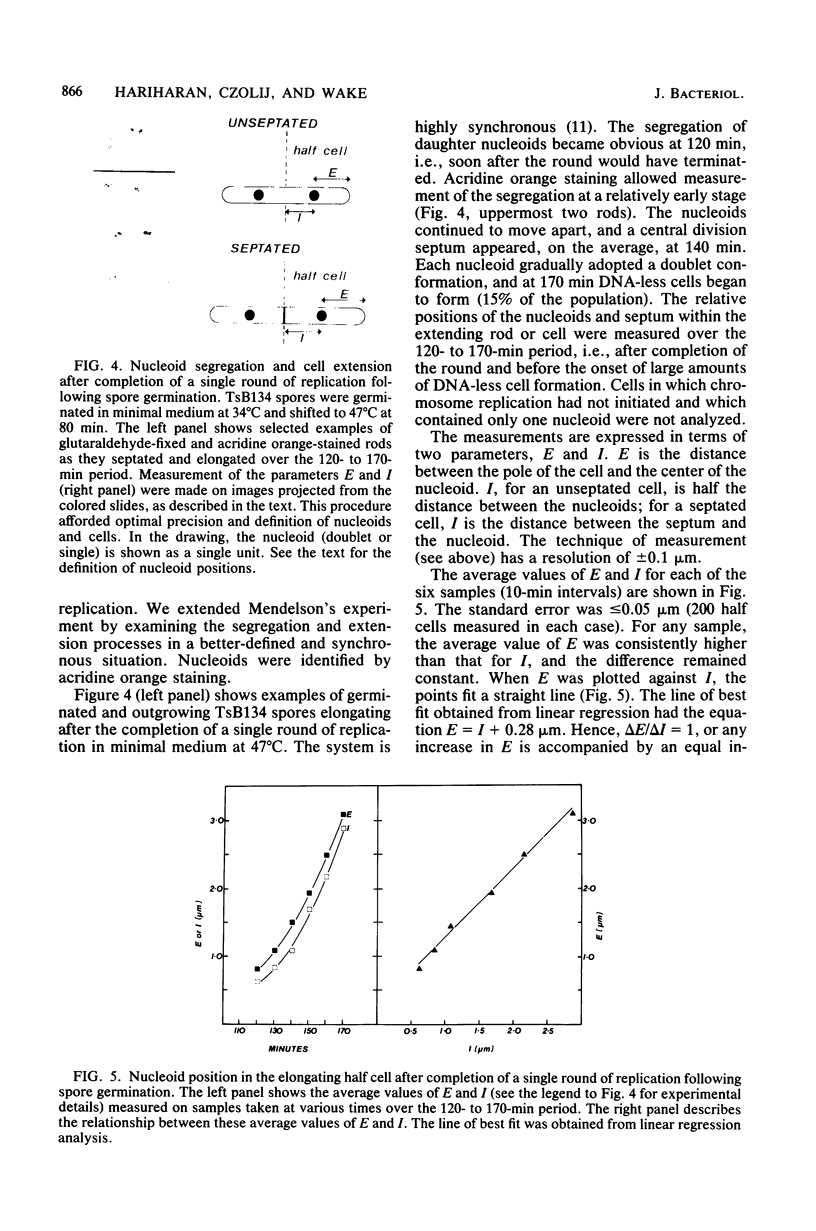
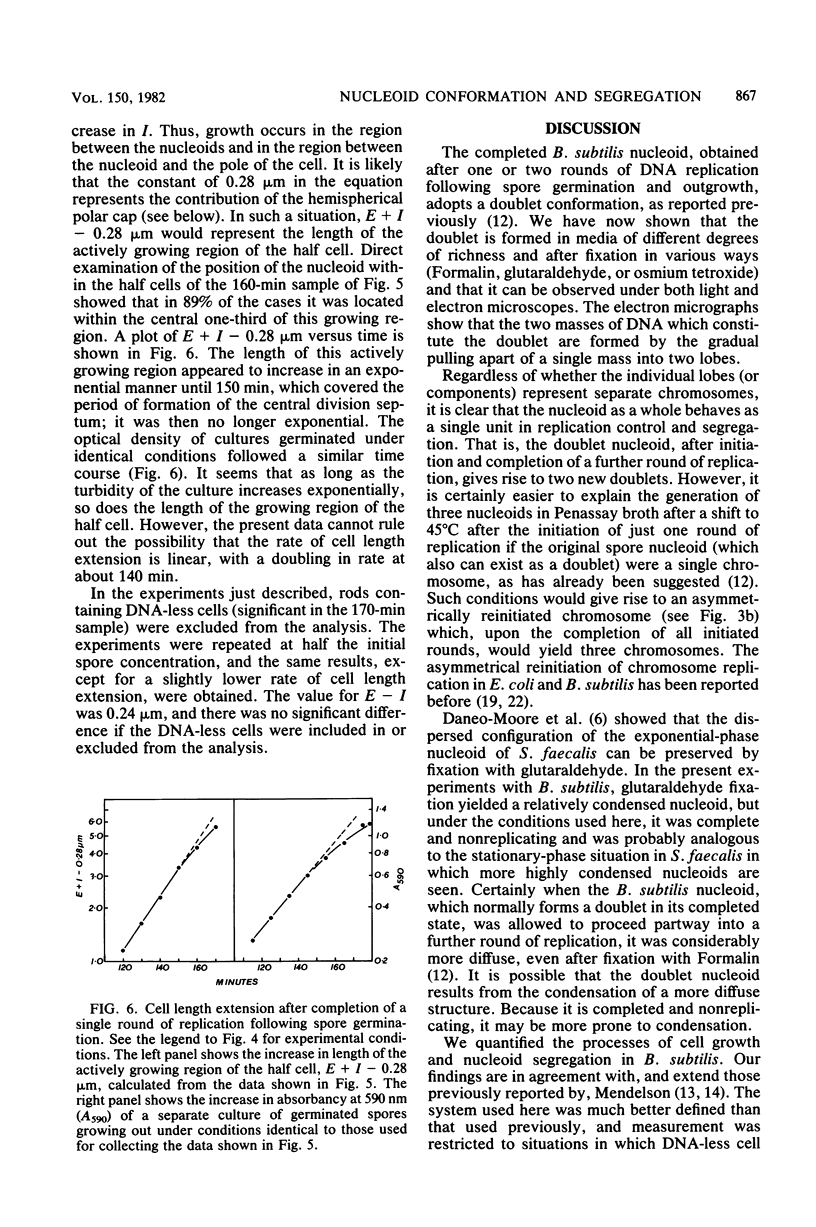
When germinating spores of the temperature-sensitive DNA initiation mutant of Bacillus subtilis TsB134 are shifted to the restrictive temperature at a time such that just one or two rounds of replication are accomplished, the completed, nonreplicating nucleoids that form eventually adopt a doublet conformation. This conformation has now been observed after fixation by glutaraldehyde or osmium tetroxide, as well as by Formalin as found previously. The doublet was observed in media of different degrees of richness and under both light and electron microscopes. Electron micrographs of serial sections through the doublet were consistent with its formation by the gradual pulling apart of a single mass of DNA into two lobes. A systematic study was made of the effect of the time of shifting from the permissive to the restrictive temperature and of the restrictive temperature used on the number of nucleoids segregating within the outgrowing rod. It was established that the doublet nucleoid behaved as a single unit in replication control and segregation in both rich and poor media. Measurement of the relative position of the two segregating nucleoids within the outgrowing rod after completion of just one round of replication yielded quantitative information on the segregation and cell length extension processes. Segregation was accompanied by cell length extension at approximately equal rates on both sides of each nucleoid. Furthermore, the data were consistent with an exponential increase in such an extension with time over the early and major portion of the period studied, but it was not possible to rule out other models of length extension.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald A. R., Coapes H. E. Bacteriophage SP50 as a marker for cell wall growth in Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1195–1206. doi: 10.1128/jb.125.3.1195-1206.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callister H., Wake R. G. Characterization and mapping of temperature-sensitive division initiation mutations of Bacillus subtilis. J Bacteriol. 1981 Feb;145(2):1042–1051. doi: 10.1128/jb.145.2.1042-1051.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callister H., Wake R. G. Completed chromosomes in thymine-requiring Bacillus subtilis spores. J Bacteriol. 1974 Nov;120(2):579–582. doi: 10.1128/jb.120.2.579-582.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callister H., Wake R. G. Completion of the replication and division cycle in temperature-sensitive DNA initiation mutants of Bacillus subtilis 168 at the non-permissive temperature. J Mol Biol. 1977 Nov 25;117(1):71–84. doi: 10.1016/0022-2836(77)90023-7. [DOI] [PubMed] [Google Scholar]
- Clark D. J. The regulation of DNA replication and cell division in E. coli B-r. Cold Spring Harb Symp Quant Biol. 1968;33:823–838. doi: 10.1101/sqb.1968.033.01.094. [DOI] [PubMed] [Google Scholar]
- Daneo-Moore L., Dicker D., Higgins M. L. Structure of the nucleoid in cells of Streptococcus faecalis. J Bacteriol. 1980 Feb;141(2):928–937. doi: 10.1128/jb.141.2.928-937.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman B. E., Beckman M. M. Cell wall turnover at the hemispherical caps of Bacillus subtilis. J Bacteriol. 1974 Mar;117(3):1330–1334. doi: 10.1128/jb.117.3.1330-1334.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Okada Y. Chromosome segregation in an asymmetrically dividing bacterium, Caulobacter crescentus. J Mol Biol. 1980 Jun 5;139(4):733–739. doi: 10.1016/0022-2836(80)90058-3. [DOI] [PubMed] [Google Scholar]
- McGinness T., Wake R. G. Completed Bacillus subtilis nucleoid as a doublet structure. J Bacteriol. 1979 Nov;140(2):730–733. doi: 10.1128/jb.140.2.730-733.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinness T., Wake R. G. Division septation in the absence of chromosome termination in Bacillus subtilis. J Mol Biol. 1979 Oct 25;134(2):251–264. doi: 10.1016/0022-2836(79)90035-4. [DOI] [PubMed] [Google Scholar]
- Mendelson N. H. Can defective segregation prevent initiation? Cold Spring Harb Symp Quant Biol. 1968;33:313–316. doi: 10.1101/sqb.1968.033.01.035. [DOI] [PubMed] [Google Scholar]
- Mendelson N. H. Nuclear segregation without DNA replication in Bacillus subtilis. Biochim Biophys Acta. 1969 Sep 17;190(1):132–138. doi: 10.1016/0005-2787(69)90162-2. [DOI] [PubMed] [Google Scholar]
- Oishi M., Oishi A., Sueoka N. Location of genetic loci of soluble RNA on Bacillus subtilis chromosome. Proc Natl Acad Sci U S A. 1966 May;55(5):1095–1103. doi: 10.1073/pnas.55.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Nuclear segregation in Bacillus subtilis. Nature. 1974 Jul 19;250(463):252–254. doi: 10.1038/250252a0. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Surface extension and the cell cycle in prokaryotes. Adv Microb Physiol. 1978;18:105–176. doi: 10.1016/s0065-2911(08)60416-6. [DOI] [PubMed] [Google Scholar]
- Van Ness J., Pettijohn D. E. A simple autoradiographic method for investigating long range chromosome substructure: size and number of DNA molecules in isolated nucleoids of Escherichia coli. J Mol Biol. 1979 Apr 15;129(3):501–508. doi: 10.1016/0022-2836(79)90509-6. [DOI] [PubMed] [Google Scholar]
- Wake R. G. Visualization of reinitiated chromosomes in Bacillus subtilis. J Mol Biol. 1972 Jul 28;68(3):501–509. doi: 10.1016/0022-2836(72)90102-7. [DOI] [PubMed] [Google Scholar]
- Winston S., Sueoka N. DNA-membrane association is necessary for initiation of chromosomal and plasmid replication in Bacillus subtilis. Proc Natl Acad Sci U S A. 1980 May;77(5):2834–2838. doi: 10.1073/pnas.77.5.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A. Induction of chromosome re-initiations in a thermosensitive DNA mutant of Escherichiacoli. J Mol Biol. 1970 Sep 14;52(2):371–386. doi: 10.1016/0022-2836(70)90037-9. [DOI] [PubMed] [Google Scholar]
- de Chastellier C., Frehel C., Ryter A. Cell wall growth of Bacillus megaterium: cytoplasmic radioactivity after pulse-labeling with tritiated diaminopimelic acid. J Bacteriol. 1975 Sep;123(3):1197–1207. doi: 10.1128/jb.123.3.1197-1207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]